Unknown salt calibration curve 10- The calibration curve was generated using known concentrations of five solutions of a newly discovered inorganic salt. The density value of each solution was determined and the curve with its equation is shown in the 9. y=1.11x+0.203 graph. A student is asked to analyze a solution of this salt with a density of 2.00 g/mL. What is the concentration, in % 7- (w/v), of the unknown salt solution? 5- 4 salt concentration = % (w/v) 2- - TOOLS Density (g/mL)
Unknown salt calibration curve 10- The calibration curve was generated using known concentrations of five solutions of a newly discovered inorganic salt. The density value of each solution was determined and the curve with its equation is shown in the 9. y=1.11x+0.203 graph. A student is asked to analyze a solution of this salt with a density of 2.00 g/mL. What is the concentration, in % 7- (w/v), of the unknown salt solution? 5- 4 salt concentration = % (w/v) 2- - TOOLS Density (g/mL)
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question
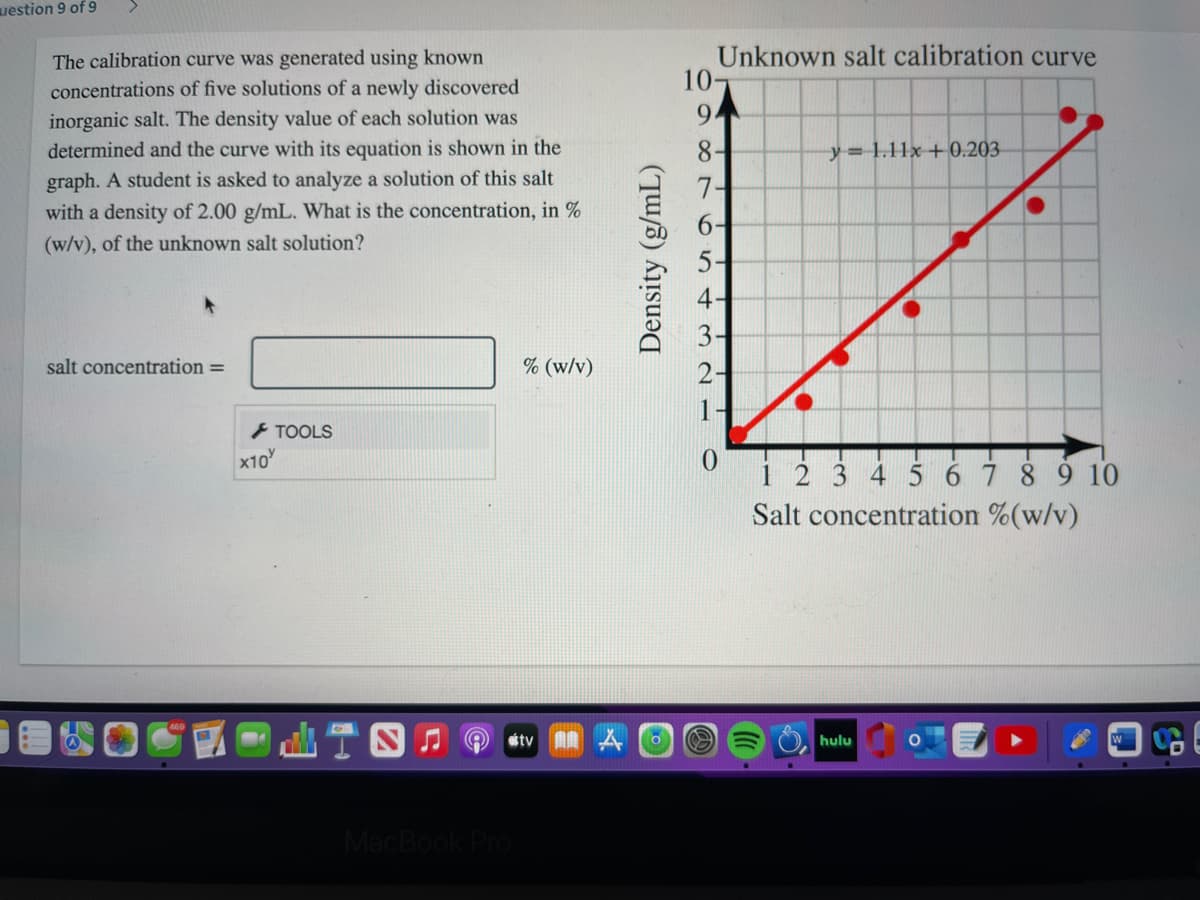
Transcribed Image Text:uestion 9 of 9
Unknown salt calibration curve
10-
The calibration curve was generated using known
concentrations of five solutions of a newly discovered
inorganic salt. The density value of each solution was
9.
determined and the curve with its equation is shown in the
8-
y= 1.11x+ 0.203
graph. A student is asked to analyze a solution of this salt
with a density of 2.00 g/mL. What is the concentration, in %
7-
(w/v), of the unknown salt solution?
5-
salt concentration =
% (w/v)
} TOOLS
x10
i 2 3 4 5 67 8 9 10
Salt concentration %(w/v)
山T四
stv A A
hulu
MacBook
Density (g/mL)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning