● ● Calculate the amount of each chemical required to make a 10x stock of TBE in a final volume of 150 mL (Table 7) Molecular weight and final concentration (mM) are given. The chemicals are dissolved in distilled water Table 7 Preparing 10x TBE Component Tris Base Boric Acid Molecular Weight or Concentration 121.14 g/mol 1 M 10x Stock Amount (grams or mL) needed for 150 mL Stock Concentration to make 890 mM 890 mM Final 1x Working Concentration to run gel 89 mM 89 mM
● ● Calculate the amount of each chemical required to make a 10x stock of TBE in a final volume of 150 mL (Table 7) Molecular weight and final concentration (mM) are given. The chemicals are dissolved in distilled water Table 7 Preparing 10x TBE Component Tris Base Boric Acid Molecular Weight or Concentration 121.14 g/mol 1 M 10x Stock Amount (grams or mL) needed for 150 mL Stock Concentration to make 890 mM 890 mM Final 1x Working Concentration to run gel 89 mM 89 mM
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.29QAP
Related questions
Question
100%
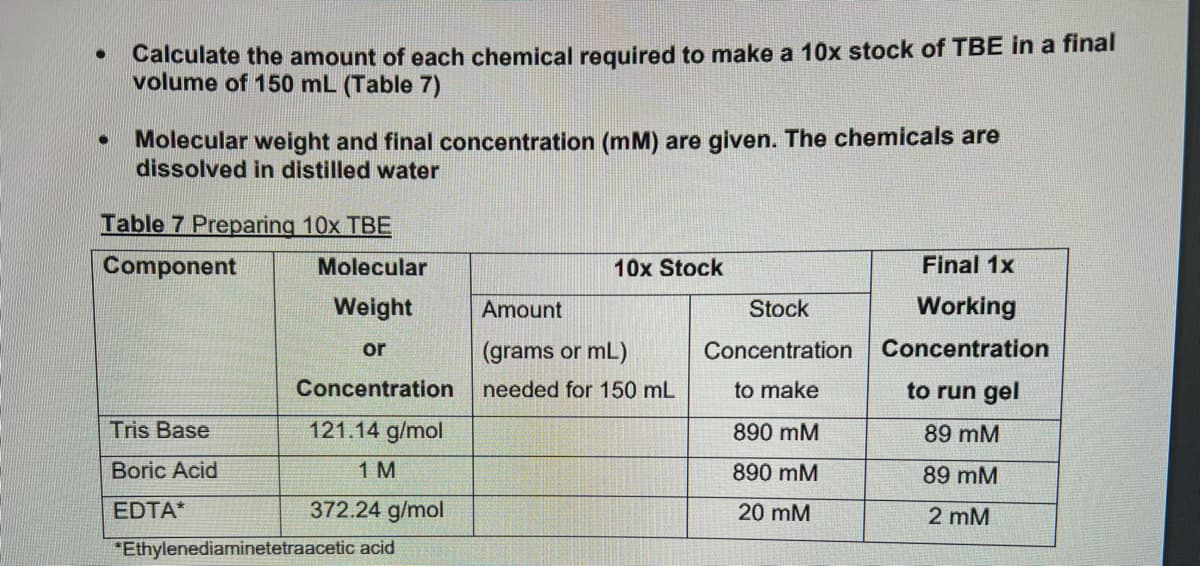
Transcribed Image Text:Calculate the amount of each chemical required to make a 10x stock of TBE in a final
volume of 150 mL (Table 7)
Molecular weight and final concentration (mM) are given. The chemicals are
dissolved in distilled water
Table 7 Preparing 10x TBE
Component
Tris Base
Boric Acid
EDTA*
Molecular
Weight
or
Concentration
121.14 g/mol
1 M
372.24 g/mol
*Ethylenediaminetetraacetic acid
10x Stock
Amount
(grams or mL)
needed for 150 mL
Stock
Concentration
to make
890 mM
890 mM
20 mM
Final 1x
Working
Concentration
to run gel
89 mM
89 mM
2 mM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning