Use the emission spectra below to answer the following questions. D, D sepnajs17a SUN |434 410,1 700 Hydrogen H 500 400 01.5 2.1 1471a 47,1 402.6 700 500 400 Helum He 6234 j615,2 546,1 pa2.s 435 Lveollezor Mercury Ho 700 600 500 400 Uranium U After graphing E vs you obtain the following best-fit linear line: y = -1.95x + 5.01. Calculate RH from the slope of the line. ninitial Note: These numbers are make up given the limitations of Canvas. Your numbers in lab will be different. Report your answer to two places after the decimal.
Use the emission spectra below to answer the following questions. D, D sepnajs17a SUN |434 410,1 700 Hydrogen H 500 400 01.5 2.1 1471a 47,1 402.6 700 500 400 Helum He 6234 j615,2 546,1 pa2.s 435 Lveollezor Mercury Ho 700 600 500 400 Uranium U After graphing E vs you obtain the following best-fit linear line: y = -1.95x + 5.01. Calculate RH from the slope of the line. ninitial Note: These numbers are make up given the limitations of Canvas. Your numbers in lab will be different. Report your answer to two places after the decimal.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter13: Introduction To Symmetry In Quantum Mechanics
Section: Chapter Questions
Problem 13.11E
Related questions
Question
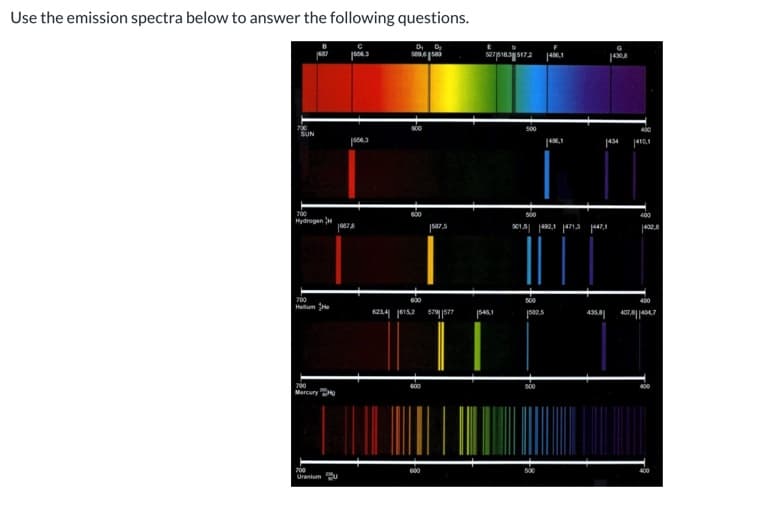
Transcribed Image Text:Use the emission spectra below to answer the following questions.
D, D
sepnajs17a
SUN
|434
410,1
700
Hydrogen H
500
400
01.5 2.1 1471a 47,1
402.6
700
500
400
Helum He
6234 j615,2
546,1
pa2.s
435
Lveollezor
Mercury Ho
700
600
500
400
Uranium U

Transcribed Image Text:After graphing E vs
you obtain the following best-fit linear line: y = -1.95x + 5.01. Calculate RH from the slope of the line.
ninitial
Note: These numbers are make up given the limitations of Canvas. Your numbers in lab will be different. Report your answer to two places after
the decimal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning