Use the graph below to answer the following questions. Assume one atom produces one count per minute. Do not include units in your answers. 80 70 60 50 40 30 20 10 2. 4. Time (Days) a. How many atoms are in the original sample size of this radioisotope? b. How long is the half-life in days? c. How many atoms are left after 2 half-lives? Counts per minute
Use the graph below to answer the following questions. Assume one atom produces one count per minute. Do not include units in your answers. 80 70 60 50 40 30 20 10 2. 4. Time (Days) a. How many atoms are in the original sample size of this radioisotope? b. How long is the half-life in days? c. How many atoms are left after 2 half-lives? Counts per minute
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter19: Radioactivity And Nuclear Energy
Section19.2: Application Of Radioactivity
Problem 4RQ
Related questions
Question
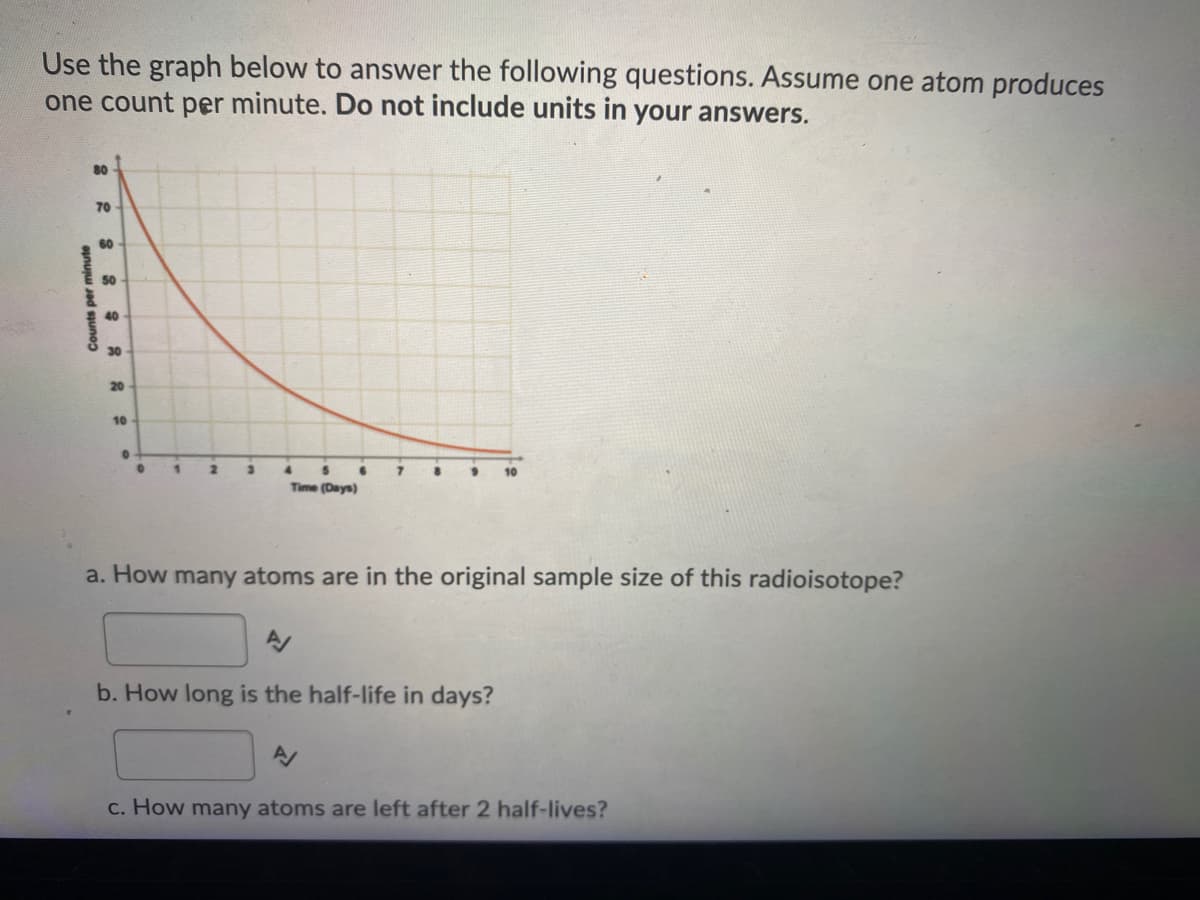
Transcribed Image Text:Use the graph below to answer the following questions. Assume one atom produces
one count per minute. Do not include units in your answers.
80
70
60
50
40
30
20
10
4.
Time (Days)
a. How many atoms are in the original sample size of this radioisotope?
b. How long is the half-life in days?
c. How many atoms are left after 2 half-lives?
Counts per minute
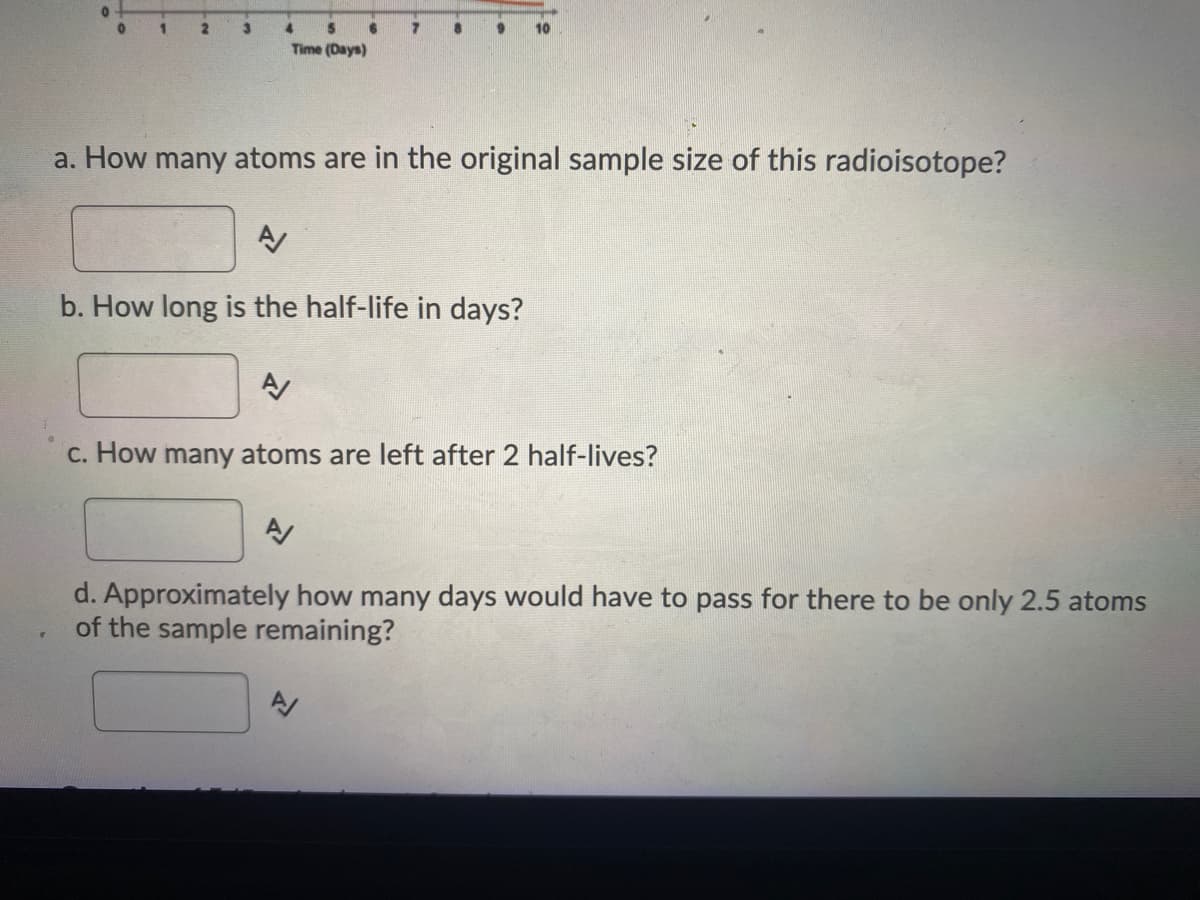
Transcribed Image Text:Time (Days)
a. How many atoms are in the original sample size of this radioisotope?
b. How long is the half-life in days?
c. How many atoms are left after 2 half-lives?
d. Approximately how many days would have to pass for there to be only 2.5 atoms
of the sample remaining?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning