Use the molar bond enthalpy data in the table to estimate the Average molar bond enthalpies (H bond) value of AHixn for the equation Bond kJ-mol¬1 Bond kJ-mol-1 CCI,(g) + 2 F,(g) CF (g) + 2 Cl,(g) О-Н 464 C=N 890 0–0 142 N-H 390 The bonding in the molecules is shown. С-О 351 N-N 159 F O=0 502 N=N 418 CI-CI F-F + C=0 730 N=N 945 CI- F-F -CI С-С 347 F-F 155 C=C 615 Cl–CI 243 C=C 811 Br-Br 192 С-Н 414 Н-Н 435 C-F 439 Н-F 565 C-CI 331 Н-СІ 431 С-Br 276 Н-Br 368 С-N 293 H-S 364 C=N 615 S-S 225 AH: kL.mol-!
Use the molar bond enthalpy data in the table to estimate the Average molar bond enthalpies (H bond) value of AHixn for the equation Bond kJ-mol¬1 Bond kJ-mol-1 CCI,(g) + 2 F,(g) CF (g) + 2 Cl,(g) О-Н 464 C=N 890 0–0 142 N-H 390 The bonding in the molecules is shown. С-О 351 N-N 159 F O=0 502 N=N 418 CI-CI F-F + C=0 730 N=N 945 CI- F-F -CI С-С 347 F-F 155 C=C 615 Cl–CI 243 C=C 811 Br-Br 192 С-Н 414 Н-Н 435 C-F 439 Н-F 565 C-CI 331 Н-СІ 431 С-Br 276 Н-Br 368 С-N 293 H-S 364 C=N 615 S-S 225 AH: kL.mol-!
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 52P
Related questions
Question
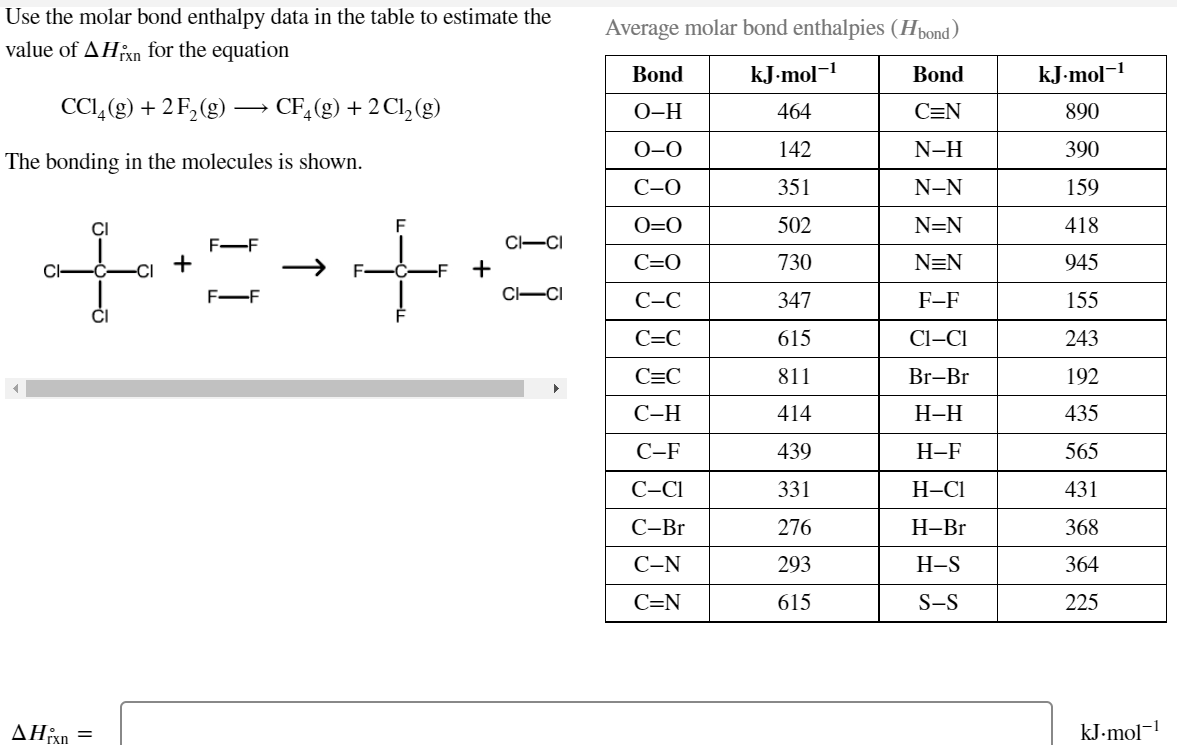
Transcribed Image Text:Use the molar bond enthalpy data in the table to estimate the
Average molar bond enthalpies (Hpond)
value of AHixn for the equation
Bond
kJ-mol-
Bond
kJ-mol-
CCI,(g) + 2F,(g) –→ CF,(g) + 2Cl,(g)
О-Н
464
C=N
890
0-0
142
N-H
390
The bonding in the molecules is shown.
С-О
351
N-N
159
CI
O=0
502
N=N
418
CI-CI
F-F
+
C=0
730
N=N
945
Cl-
F-F
CI-CI
С-С
347
F-F
155
C=C
615
Cl-CI
243
C=C
811
Br-Br
192
С-Н
414
Н-Н
435
С-F
439
Н-F
565
C-CI
331
Н-СІ
431
С-Br
276
Н-Br
368
С-N
293
Н-S
364
C=N
615
S-S
225
ΔΗΚ
kJ-mol-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning