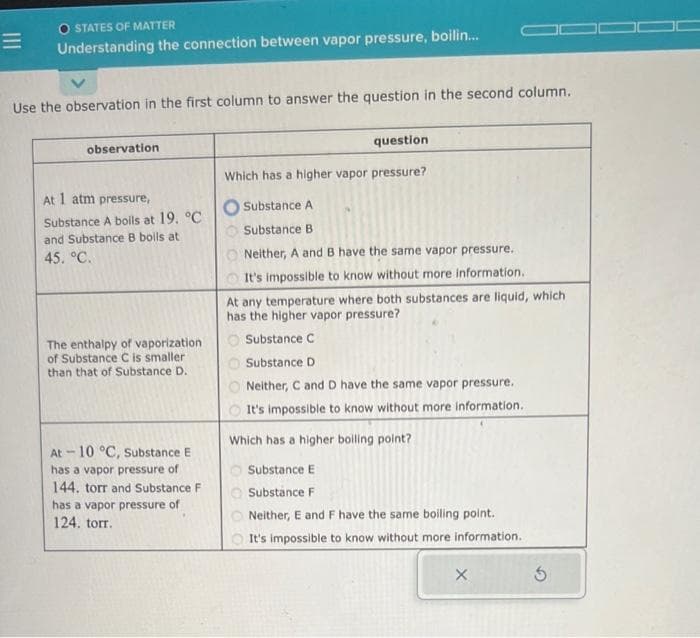
Use the observation in the first column to answer the question in the second column. observation At 1 atm pressure, Substance A boils at 19. °C and Substance B boils at 45. °C. The enthalpy of vaporization of Substance C is smaller than that of Substance D. At-10 °C, Substance E has a vapor pressure of 144. torr and Substance F has a vapor pressure of 124. torr. question Which has a higher vapor pressure? O Substance A Substance B Neither, A and B have the same vapor pressure. It's impossible to know without more information. At any temperature where both substances are liquid, which has the higher vapor pressure? O Substance C Substance D Neither, C and D have the same vapor pressure. It's impossible to know without more information. Which has a higher boiling point? Substance E Substance F Neither, E and F have the same boiling point. It's impossible to know without more information. X 5
Use the observation in the first column to answer the question in the second column. observation At 1 atm pressure, Substance A boils at 19. °C and Substance B boils at 45. °C. The enthalpy of vaporization of Substance C is smaller than that of Substance D. At-10 °C, Substance E has a vapor pressure of 144. torr and Substance F has a vapor pressure of 124. torr. question Which has a higher vapor pressure? O Substance A Substance B Neither, A and B have the same vapor pressure. It's impossible to know without more information. At any temperature where both substances are liquid, which has the higher vapor pressure? O Substance C Substance D Neither, C and D have the same vapor pressure. It's impossible to know without more information. Which has a higher boiling point? Substance E Substance F Neither, E and F have the same boiling point. It's impossible to know without more information. X 5
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.87PAE: 8.87 Use the vapor pressure curves illustrated here to answer the questions that follow. (a) What is...
Related questions
Question
Transcribed Image Text:O STATES OF MATTER
Understanding the connection between vapor pressure, boilin...
Use the observation in the first column to answer the question in the second column.
observation
At 1 atm pressure,
Substance A boils at 19. °C
and Substance B boils at
45. °C.
The enthalpy of vaporization
of Substance C is smaller
than that of Substance D.
At -10 °C, Substance E
has a vapor pressure of
144. torr and Substance F
has a vapor pressure of
124. torr.
question
Which has a higher vapor pressure?
Substance A
Substance B
Neither, A and B have the same vapor pressure.
It's impossible to know without more information.
At any temperature where both substances are liquid, which
has the higher vapor pressure?
Substance C
Substance D
Neither, C and D have the same vapor pressure.
It's impossible to know without more information.
Which has a higher boiling point?
Substance E
Substance F
Neither, E and F have the same boiling point.
It's impossible to know without more information.
X
5
Transcribed Image Text:E
STATES OF MATTER
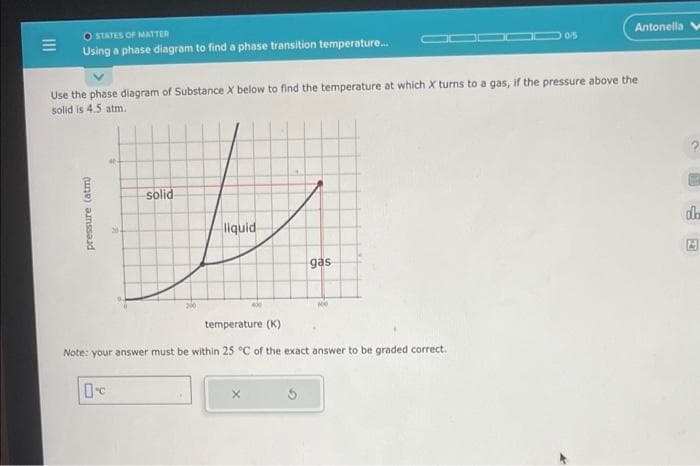
Using a phase diagram to find a phase transition temperature...
pressure (atm)
Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the
solid is 4.5 atm.
solid
t
liquid
0
gas
temperature (K)
Note: your answer must be within 25 °C of the exact answer to be graded correct.
5
100
10/5
Antonella V
?
dh
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning