Use the observation in the first column to answer the question in the second column. observation question Which has the higher boiling point? Substance E The enthalpy of vaporization of Substance E is smaller Substance F than that of Substance F. Neither, E and F have the same boiling point. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 1 atm pressure, Substance C Substance C boils at 142. °C and Substance D Substance D boils at 130. °C. Neither, C and D have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher boiling point? At 94 °C, Substance A has a vapor pressure of 123. torr and Substance B Substance A Substance B has a vapor pressure of 143. torr. Neither, A and B have the same boiling point. It's impossible to know without more information.
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
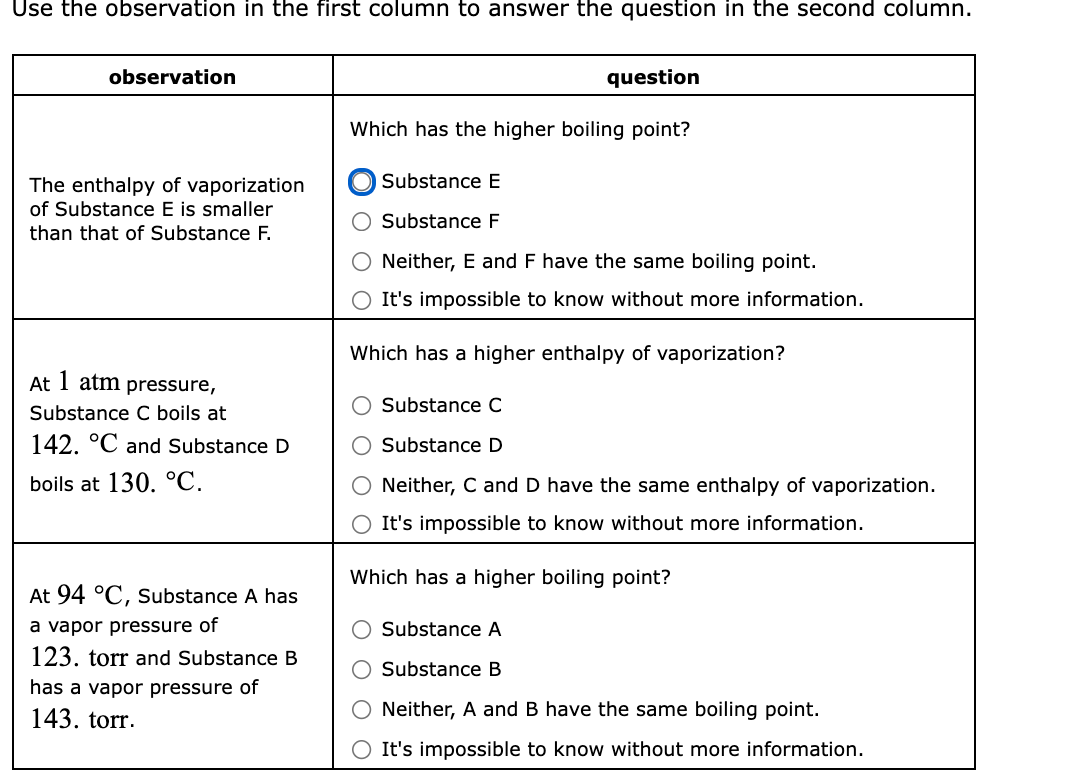
Here we have to relate the Enthalpy of vapourisation with boiling point of a substance.Enthaly of vapourisation is the heat absorbed when a substance starts boiling .
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images









