Use the pHet simulation Isotopes to determine 2 stable isotopes for 3 of the above elements listed in the table. Use the nuclear notation to denote each stable isotope. A. B. C. D. E. F. Provide Common Ions for Zn and Fe, explain their valence electron situation and why Fe has more common ions than Zn.
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
No Plagiarism Please!
|
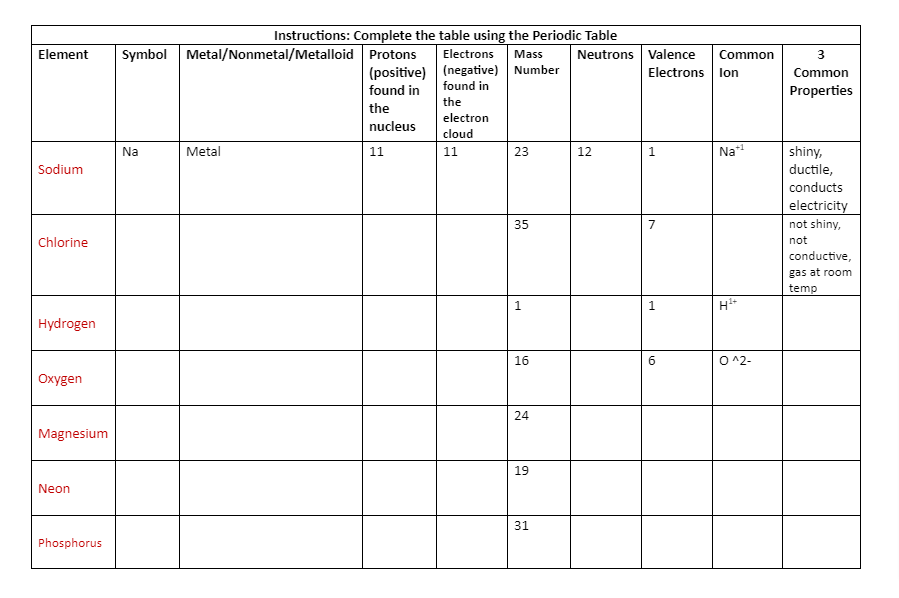
Instructions: Complete the table using the Periodic Table |
|||||||||
|
Element |
Symbol |
Metal/Nonmetal/Metalloid |
Protons (positive) found in the nucleus |
Electrons (negative) found in the electron cloud |
Mass Number |
Neutrons |
Valence Electrons |
Common Ion |
3 Common Properties |
Sodium |
Na |
Metal |
11 |
11 |
23 |
12 |
1 |
Na+1 |
shiny, ductile, conducts electricity |
Chlorine |
35 |
7 |
not shiny, not conductive, gas at room temp |
||||||
Hydrogen |
1 |
1 |
H1+ |
||||||
Oxygen |
16 |
6 |
O ^2- |
||||||
Magnesium |
24 |
||||||||
Neon |
19 |
||||||||
Phosphorus |
31 |
- Use the pHet simulation Isotopes to determine 2 stable isotopes for 3 of the above elements listed in the table. Use the nuclear notation to denote each stable isotope. A. B. C. D. E. F.
- Provide Common Ions for Zn and Fe, explain their valence electron situation and why Fe has more common ions than Zn.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps









