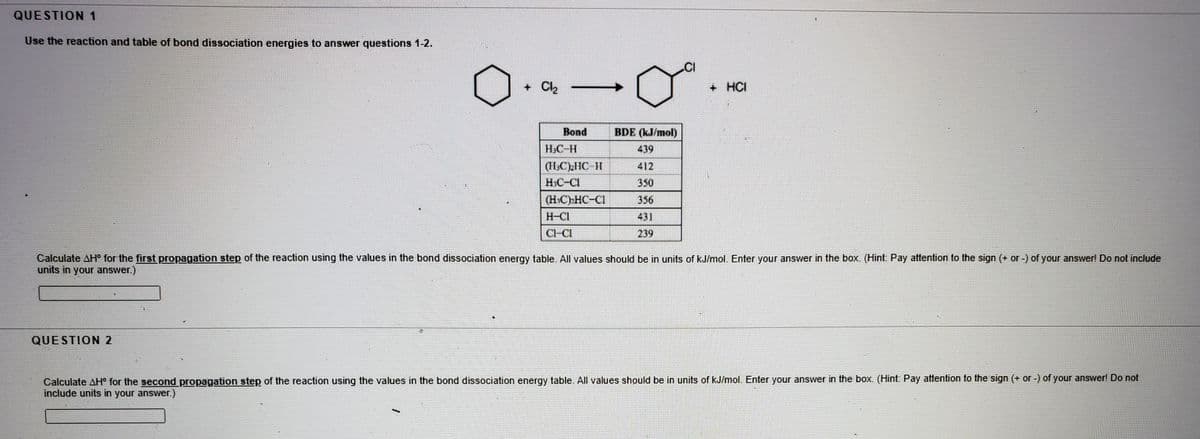
Use the reaction and table of bond dissociation energies to answer questions 1-2. + Ch + HCI Воnd BDE (kJ/mol) HC-H 439 412 HC-CI 350 (HC)-HC-CI 356 H-CI 431 CHCI 239 Calculate AH for the first propagation step of the reaction using the values in the bond dissociation energy table. All values should be in units of kJ/mol. Enter your answer in the box. (Hint: Pay attention to the sign (+ or -) of your answer! Do not include units in your answer.) QUESTION 2 Calculate AH for the second propagation step of the reaction using the values in the bond dissociation energy table. All values should be in units of kJ/mol. Enter your answer in the box. (Hint: Pay attention to the sign (+ or -) of your answer! Do not include units in your answer.)
Use the reaction and table of bond dissociation energies to answer questions 1-2. + Ch + HCI Воnd BDE (kJ/mol) HC-H 439 412 HC-CI 350 (HC)-HC-CI 356 H-CI 431 CHCI 239 Calculate AH for the first propagation step of the reaction using the values in the bond dissociation energy table. All values should be in units of kJ/mol. Enter your answer in the box. (Hint: Pay attention to the sign (+ or -) of your answer! Do not include units in your answer.) QUESTION 2 Calculate AH for the second propagation step of the reaction using the values in the bond dissociation energy table. All values should be in units of kJ/mol. Enter your answer in the box. (Hint: Pay attention to the sign (+ or -) of your answer! Do not include units in your answer.)
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.8: Oxidation Of Aromatic Compounds
Problem 19P
Related questions
Question
Question 2 needed
Transcribed Image Text:QUESTION 1
Use the reaction and table of bond dissociation energies to answer questions 1-2.
+ Cl2
+ HCI
Bond
BDE (kJ/mol)
H.C-H
439
(HC)IC 11
HIC-CI
412
350
(H.C)»HC-CI
356
H-CI
431
Cl-CI
239
Calculate AH° for the first propagation step of the reaction using the values in the bond dissociation energy table. All values should be in units of kJ/mol. Enter your answer in the box. (Hint: Pay attention to the sign (+ or -) of your answer! Do not include
units in your answer.)
QUESTION 2
Calculate AH° for the second propagation step of the reaction using the values in the bond dissociation energy table. All values should be in units of kJ/mol. Enter your answer in the box. (Hint: Pay attention to the sign (+ or -) of your answer! Do not
include units in your answer.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning