Use the References to access impertant values if needed for this question. In the laboratory you are given the task of separating Ag" and Co" ions in aqueous solution. For each reagent listed below indicate if it can be used to separate the ions. Type "Y" for yes or "N" for no. If the reagent CAN be use to separate the ions, give the formula of the precipitate. If it cannot, type "No" Y or N Reagent Formula of Precipitate if YES 1. Nal K2S 3. K2SO4 Submit Answer Retry Entire Group 3 more group attempts remaining
Use the References to access impertant values if needed for this question. In the laboratory you are given the task of separating Ag" and Co" ions in aqueous solution. For each reagent listed below indicate if it can be used to separate the ions. Type "Y" for yes or "N" for no. If the reagent CAN be use to separate the ions, give the formula of the precipitate. If it cannot, type "No" Y or N Reagent Formula of Precipitate if YES 1. Nal K2S 3. K2SO4 Submit Answer Retry Entire Group 3 more group attempts remaining
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 86AP
Related questions
Question
12b.
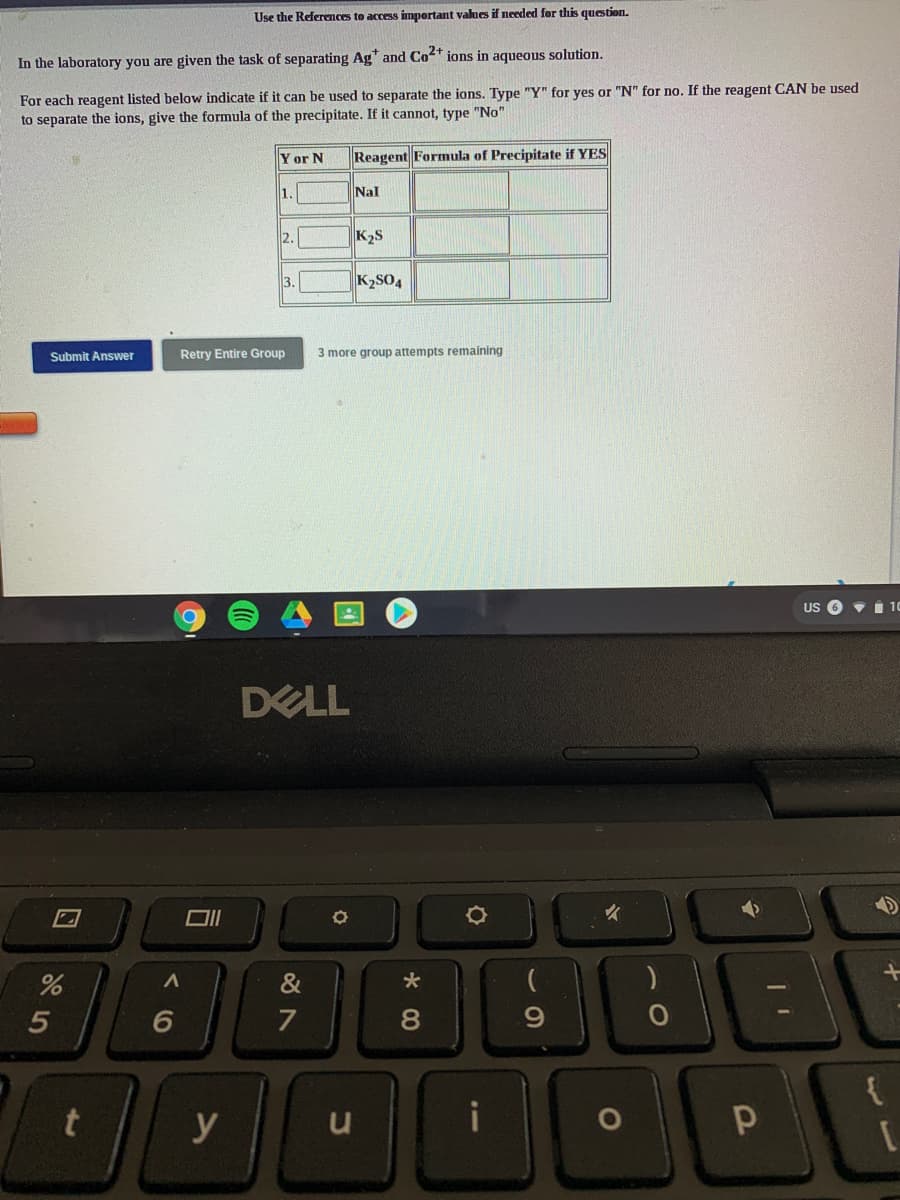
Transcribed Image Text:Use the References to access impertant values if needed for this question.
In the laboratory you are given the task of separating Ag* and Co2+ ions in aqueous solution.
For each reagent listed below indicate if it can be used to separate the ions. Type "Y" for yes or "N" for no. If the reagent CAN be used
to separate the ions, give the formula of the precipitate. If it cannot, type "No"
Y or N
Reagent Formula of Precipitate if YES
1.
Nal
2.
K2S
3.
K2SO4
Submit Answer
Retry Entire Group
3 more group attempts remaining
US O v i 10
DELL
&
5
7
8.
{
y
くO
口
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
