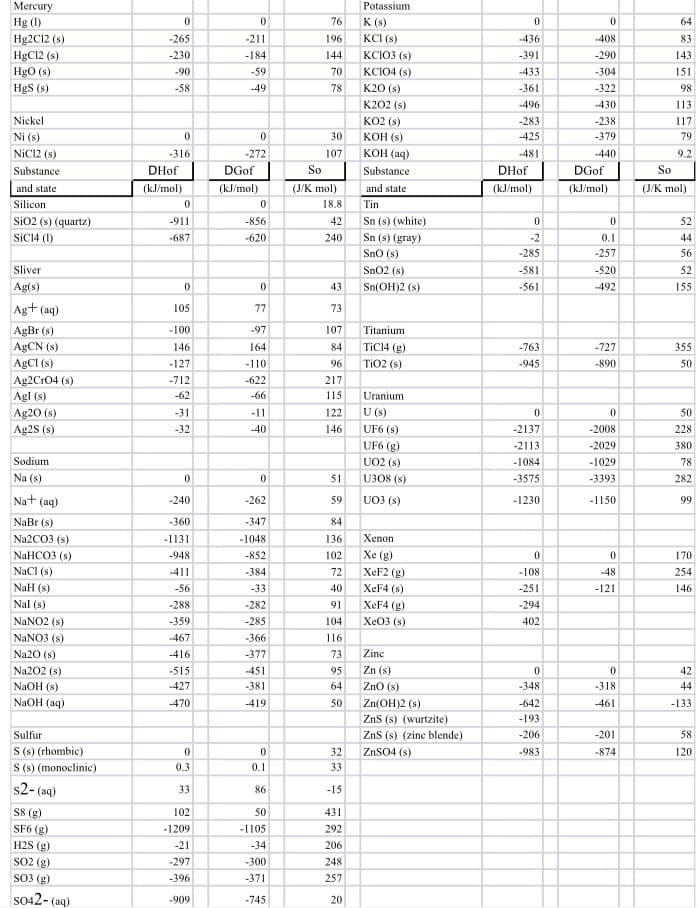
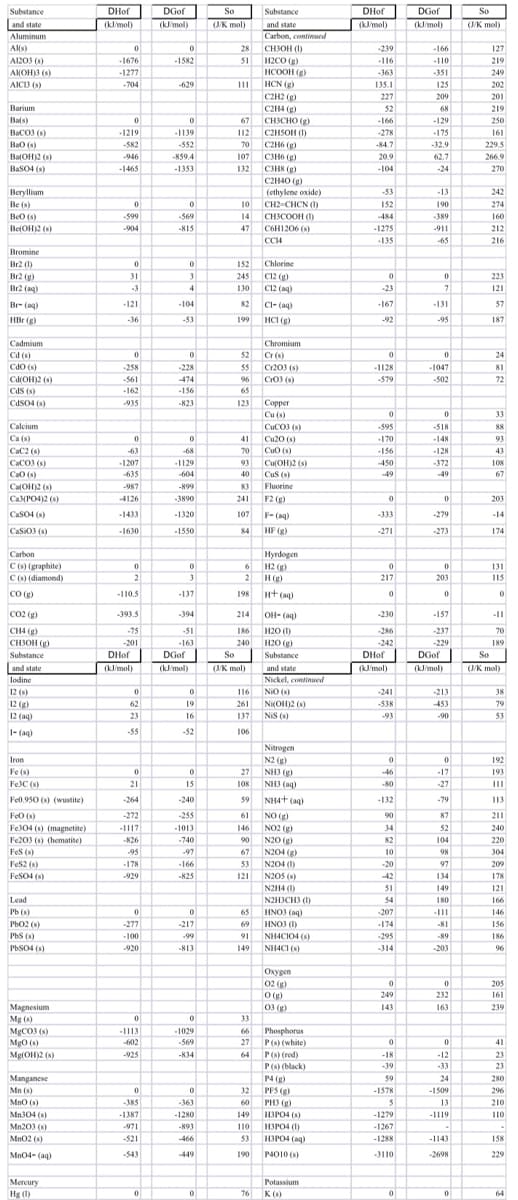
Use thermodynamic values in the table provided to calculate the ΔG for the following process in two ways. 4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (g) at 25˚C a) Use Free Energy values to calculate ΔG. b) Use Enthalpy and Entropy values with Gibbs Equation to calculate ΔG. c) What do your final ΔG values tell us about this reaction?
Use thermodynamic values in the table provided to calculate the ΔG for the following process in two ways. 4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (g) at 25˚C a) Use Free Energy values to calculate ΔG. b) Use Enthalpy and Entropy values with Gibbs Equation to calculate ΔG. c) What do your final ΔG values tell us about this reaction?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter15: Molecular Luminescence Spectrometry
Section: Chapter Questions
Problem 15.12QAP: Equations for the chemiluminescence determination of SO2 are given on page 383. Derive an expression...
Related questions
Question
Use thermodynamic values in the table provided to calculate the ΔG for the following process in two ways.
4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (g) at 25˚C
a) Use Free Energy values to calculate ΔG.
b) Use Enthalpy and Entropy values with Gibbs Equation to calculate ΔG.
c) What do your final ΔG values tell us about this reaction?
Transcribed Image Text:Mercury
Potassium
K (s)
Hg (1)
Hg2C12 (s)
76
64
-265
-211
196
KCI (s)
-436
-408
83
HgC12 (s)
Hgo (s)
HgS (s)
-230
-184
144
KCIO3 (s)
-391
-290
143
-90
-59
70
KCIO4 (s)
-433
-304
151
-58
-49
78
K20 (s)
-361
-322
98
K202 (s)
KO2 (s)
КОН (s)
КоН (ад)
-496
-430
113
Nickel
-283
-238
117
Ni (s)
30
-425
-379
79
NIC12 (s)
-316
-272
107
-481
-440
9.2
Substance
DHof
DGof
So
Substance
DHof
DGof
So
and state
(kJ/mol)
(kJ/mol)
(J/K mol)
and state
(kJ/mol)
(kJ/mol)
(J/K mol)
Silicon
18.8
Tin
SiO2 (s) (quartz)
-911
-856
42
Sn (s) (white)
52
SIC14 (1)
-687
Sn (s) (gray)
Sno (s)
-620
240
-2
0.1
44
-285
-257
56
Sliver
SnO2 (s)
-581
-520
52
Ag(s)
43
Sn(OH)2 (s)
-561
-492
155
Ag+ (aq)
105
77
73
AgBr (s)
AGCN (s)
-100
-97
107
Titanium
146
164
84
TİCI4 (g)
-763
-727
355
AgCI (s)
-127
-110
96
TIO2 (s)
-945
-890
50
Ag2Cr04 (s)
-712
-622
217
Agl (s)
-62
-66
115
Uranium
Ag20 (s)
-31
-11
122
U (s)
50
Ag2S (s)
-32
-40
146
UF6 (s)
-2137
-2008
228
UF6 (g)
-2113
-2029
380
Sodium
UO2 (s)
-1084
-1029
78
Na (s)
51
U308 (s)
-3575
-3393
282
Nat (aq)
-240
-262
59
UO3 (s)
-1230
-1150
99
NaBr (s)
-360
-347
84
Na2CO3 (s)
-1131
-1048
136
Xenon
NaHCO3 (s)
-948
-852
102
Xe (g)
170
NaCI (s)
-411
-384
72
XeF2 (g)
-108
-48
254
NaH (s)
-56
-33
40
XEF4 (s)
-251
-121
146
Nal (s)
-288
-282
91
XEF4 (g)
-294
NaNO2 (s)
-359
-285
104
Xe03 (s)
402
NANO3 (s)
-467
-366
116
Na20 (s)
-416
-377
73
Zinc
Na202 (s)
-515
-451
95
Zn (s)
42
NAOH (s)
-427
-381
64
ZnO (s)
-348
-318
44
NaOH (aq)
-470
-419
50
Zn(OH)2 (s)
-642
-461
-133
ZnS (s) (wurtzite)
-193
Sulfur
ZnS (s) (zinc blende)
-206
-201
58
S (s) (rhombic)
S (3) (monoclinic)
s2- (aq)
32
ZNSO4 (s)
-983
-874
120
0.3
0.1
33
33
86
-15
S8 (g)
SF6 (g)
102
50
431
-1209
-1105
292
H2S (g)
-21
-34
206
SO2 (g)
SO3 (g)
-297
-300
248
-396
-371
257
So42- (aq)
-909
-745
20
Transcribed Image Text:DHof DGof
Jmol) mol) UK mol)
DHof DGof
= 系 |
=日 "为
国 国
e日 =
三三
。 月 三
きe己 t= こ8:
日
因
=2 Sは
目
日
素3
年
月目
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
