Useful Constants 1.055 × 10-34 h. Js 6.626 × 10-34 9.109 x 10-31 1.602 x 10-1D 8.854 x 10-12 Js me kg C*Jm- Problem 1 Given that L=nh for Bohr's quantization condition for circular orbits and that the effective potential is defined to be
Useful Constants 1.055 × 10-34 h. Js 6.626 × 10-34 9.109 x 10-31 1.602 x 10-1D 8.854 x 10-12 Js me kg C*Jm- Problem 1 Given that L=nh for Bohr's quantization condition for circular orbits and that the effective potential is defined to be
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter7: Components Of Optical Instruments
Section: Chapter Questions
Problem 7.3QAP: The Wien displacement law states that the wavelength maximum in micrometers for blackbody radiation...
Related questions
Question
2
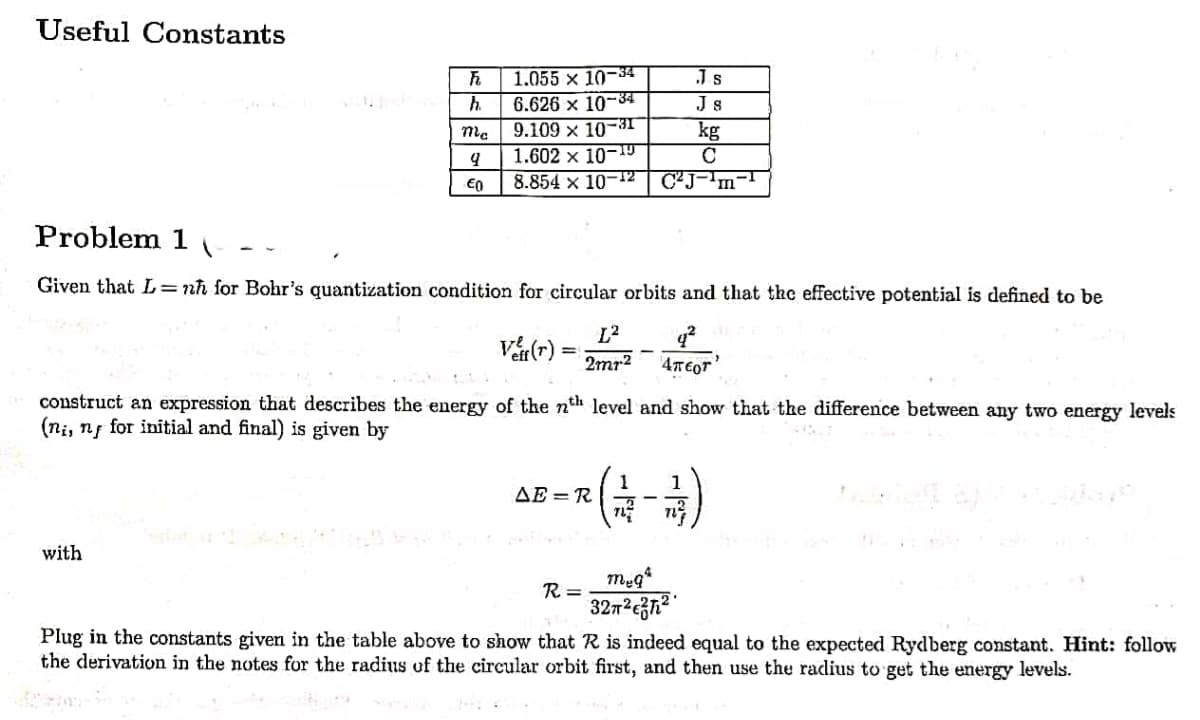
Transcribed Image Text:Useful Constants
1.055 x 10-34
6.626 x 10-34
9.109 x 10-3I
1.602 x 10-T9
8.854 x 10-12 CJ-'m-T
Js
h.
Js
kg
€0
Problem 1
Given that L= nh for Bohr's quantization condition for circular orbits and that the effective potential is defined to be
L2
Va(r) =
2mr2
construct an expression that describes the energy of the nth level and show that the difference between any two energy levels
(ni, ng for initial and final) is given by
1
AE = R
1
with
R =
32m²c3n² °
Plug in the constants given in the table above to show that R is indeed equal to the expected Rydberg constant. Hint: follow
the derivation in the notes for the radius of the circular orbit first, and then use the radius to get the energy levels.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning