Using dimensional analysis, find the theoretical mass of the Zn3(PO4)2-4H20 precipitate that should be produced when 2.19 g of Zn(C2H3O2)2•2H2O reacts completely.
Using dimensional analysis, find the theoretical mass of the Zn3(PO4)2-4H20 precipitate that should be produced when 2.19 g of Zn(C2H3O2)2•2H2O reacts completely.
Chapter13: Isolation Of Eugenol From Clov
Section: Chapter Questions
Problem 9Q
Related questions
Question
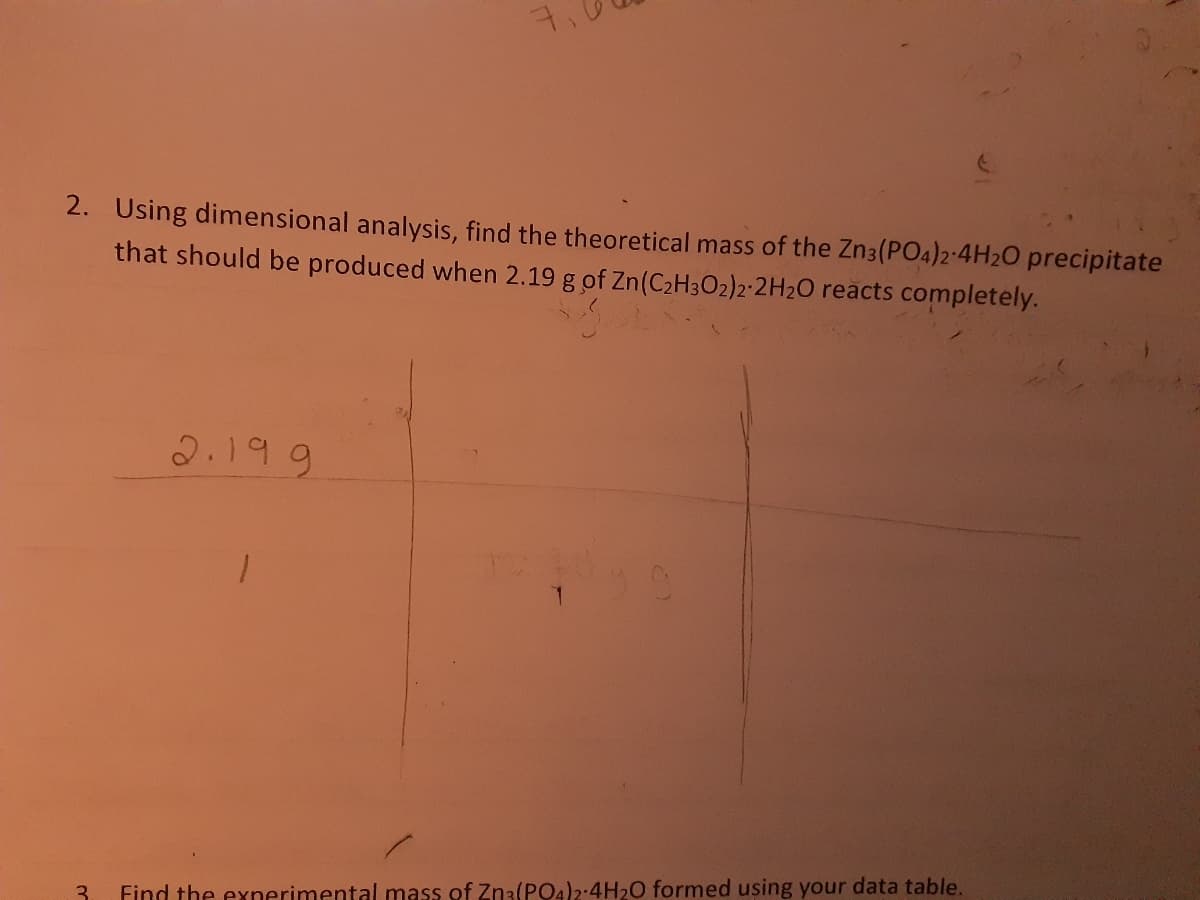
Transcribed Image Text:7.
2. Using dimensional analysis, find the theoretical mass of the Zn3(PO4)2-4H20 precipitate
that should be produced when 2.19 g of Zn(C2H3O2)2-2H2O reacts completely.
2.199
Find the exnerimental mass of Zn3(PQ4)2:4H20 formed using your data table.
Expert Solution
Step 1
Given : Mass of Zn(C2H3O2)2.2H2O reacting = 2.19 g
Molar mass of Zn(C2H3O2)2.2H2O = Atomic mass of Zn + ( Atomic mass of C X 2 + Atomic mass of H X 3 + Atomic mass of O X 2 ) X 2 + Molar mass of H2O X 2
=> Molar mass of Zn(C2H3O2)2.2H2O = 65.4 + (12 X 2 + 1 X 3 + 16 X 2) X 2 + 18 X 2 = 219.4 g/mol.
Since mass = moles X molar mass
=> 2.19 = moles of Zn(C2H3O2)2.2H2O reacting X 219.4
=> Moles of Zn(C2H3O2)2.2H2O reacting = 0.010 mol approx.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning