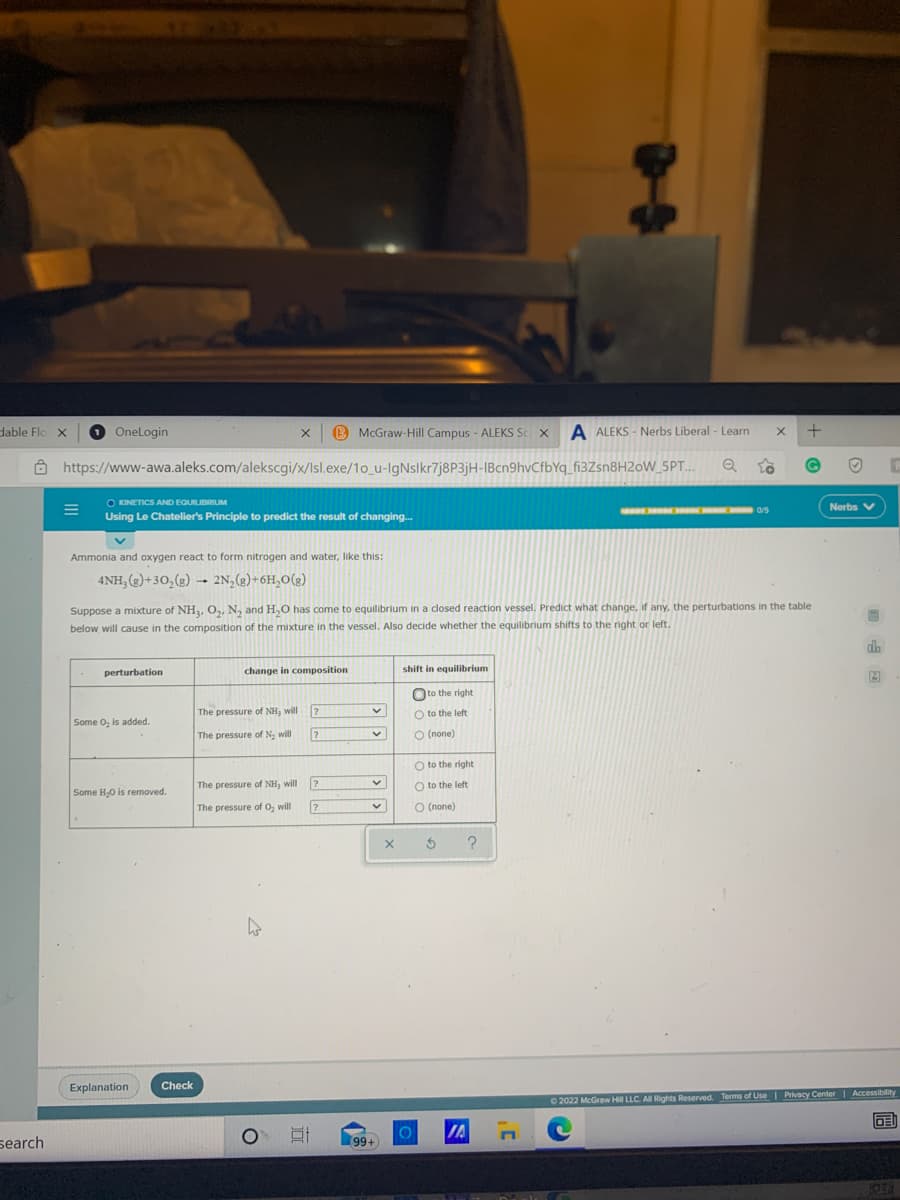
Using Le Chatelier's Principle to predict the result of changing Nerbs V Ammonia and oxygen react to form nitrogen and water, like this: 4NH, (2)+30,(2) → 2N,(e)+6H,O(2) Suppose a mixture of NH, O,, N, and H,O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the nght or left. perturbation change in composition shift in equilibrium Oto the right The pressure of NH, will Some O, is added. O to the left The pressure of N, will O (none) O to the right Some HO is removed. The pressure of NH, will 7. O to the left The pressure of O, will O (none) II
Using Le Chatelier's Principle to predict the result of changing Nerbs V Ammonia and oxygen react to form nitrogen and water, like this: 4NH, (2)+30,(2) → 2N,(e)+6H,O(2) Suppose a mixture of NH, O,, N, and H,O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the nght or left. perturbation change in composition shift in equilibrium Oto the right The pressure of NH, will Some O, is added. O to the left The pressure of N, will O (none) O to the right Some HO is removed. The pressure of NH, will 7. O to the left The pressure of O, will O (none) II
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter1: The Atom In Modern Chemistry
Section: Chapter Questions
Problem 16P: In the problem 15 above, what is vy , the y-component of the electron’s velocity, when it has...
Related questions
Question
Transcribed Image Text:dable Flo X
O Onelogin
B McGraw-Hill Campus - ALEKS Sc X
A ALEKS - Nerbs Liberal - Learn
Ô https://www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PT..
O KINETICS AND EQUILIBRIUM
Nerbs V
E a/S
Using Le Chatelier's Principle to predict the result of changing..
Ammonia and oxygen react to form nitrogen and water, like this:
4NH, (2)+30,(g) → 2N,(2)+6H,0(g)
Suppose a mixture of NH3, O,, N, and H,O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table
below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left.
perturbation
change in composition
shift in equilibrium
Oto the right
The pressure of NH3 will
O to the left
Some O, is added.
The pressure of N. will
O (none)
O to the right
The pressure of NH3 will
O to the left
Some H,O is removed.
The pressure of O, will
О (попе)
?
Explanation
Check
O 2022 McGraw Hill LLC. A Rights Reserved. Terms of Use Privacy Center Accessibility
DE
IA
search
99+
個
Expert Solution
Step 1
The problem can be solved considering Le'chatelier principal into account.
It States that" if a reaction at equalibrium is disturbed then system opposes the change in reaction and the reaction shift to that side where it can again attain equalibrium easily"
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
