Using the balanced chemical equation, determine how many mołes of NaCl will be produced, if 0.404 mol of BaCl, is allowed to react with an excess of Na,SO,. mol moles of NaCI: Using the moles of NaCl found in the previous question, determine how many grams of NaCl can be produced. mass of NaCI: Using the moles of NaCI found in a previous question, determine bow many formula units of NaCl can be produced. formula units:
Using the balanced chemical equation, determine how many mołes of NaCl will be produced, if 0.404 mol of BaCl, is allowed to react with an excess of Na,SO,. mol moles of NaCI: Using the moles of NaCl found in the previous question, determine how many grams of NaCl can be produced. mass of NaCI: Using the moles of NaCI found in a previous question, determine bow many formula units of NaCl can be produced. formula units:
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
Section: Chapter Questions
Problem 5STP
Related questions
Question
10
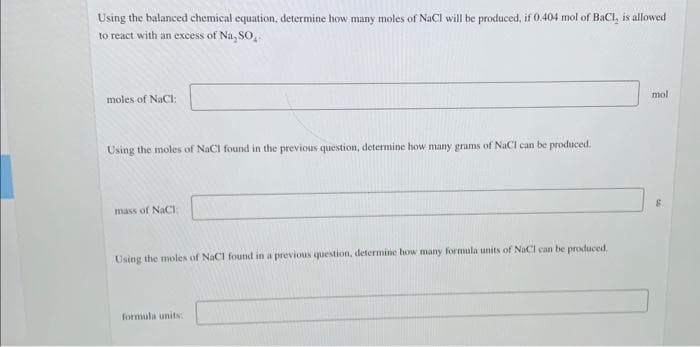
Transcribed Image Text:Using the balanced chemical equation, determine how many moles of NaCl will be produced, if 0.404 mol of BaCl, is allowed
to react with an excess of Na, SO,.
mol
moles of NaCI:
Using the moles of NaCl found in the previous question, determine how many grams of NACI can be produced.
mass of NaCI:
Using the moles of NaCI found in a previous question, determine how many formula units of NaCl can be produced.
formula units:

Transcribed Image Text:Balance the chemical equation for the reaction of BaCl, and Na, SO,.
chemical equation: BaCl, (aq) + Na, So,(aq)-
BaSO,(0)+NaCl{aq)
Which type of reaction is this?
single replacement
double replacement
decomposition
combination
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning