Using the half reactions from the given table, what is the reaction of this reduction-oxidation?
Using the half reactions from the given table, what is the reaction of this reduction-oxidation?
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter17: Metabolism: An Overview
Section: Chapter Questions
Problem 14P
Related questions
Question
100%
Can someone help with this question?
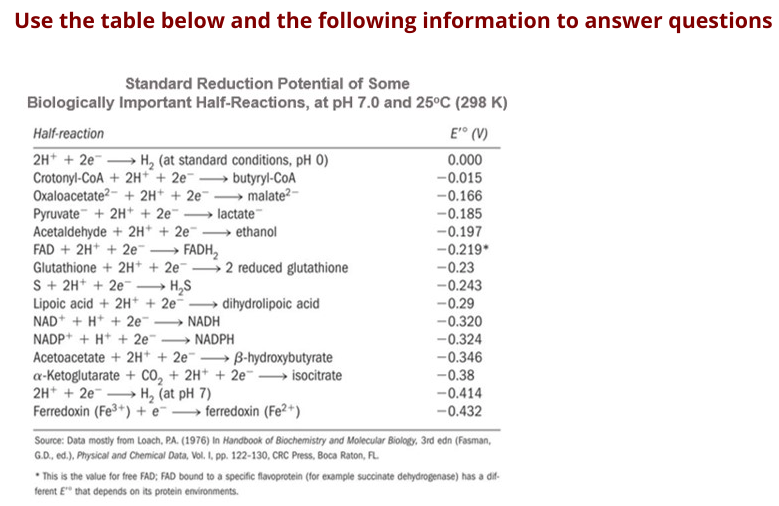
Transcribed Image Text:Use the table below and the following information to answer questions
Standard Reduction Potential of Some
Biologically Important Half-Reactions, at pH 7.0 and 25°C (298 K)
Half-reaction
E° (V)
2H* + 2e H, (at standard conditions, pH 0)
Crotonyl-CoA + 2H + 2e butyryl-CoA
Oxaloacetate?- + 2H* + 2e malate2-
Pyruvate + 2H+ + 2e
Acetaldehyde + 2H* + 2e-
FAD + 2H* + 2e .
Glutathione + 2H* + 2e 2 reduced glutathione
S + 2H+ + 2e
Lipoic acid + 2H+ + 2e
NAD* + H* + 2e
NADP+ + H* + 2e"
0.000
-0.015
-0.166
lactate
- ethanol
-0.185
-0.197
FADH,
-0.219*
>
-0.23
H,S
-0.243
→ dihydrolipoic acid
-0.29
-0.320
-0.324
-0.346
-0.38
-0.414
-0.432
NADH
- NADPH
Acetoacetate + 2H* + 2e B-hydroxybutyrate
a-Ketoglutarate + co, + 2H* + 2e → isocitrate
2H* + 2e H, (at pH 7)
Ferredoxin (Fe3+) + e ferredoxin (Fe2+)
Source: Data mostly from Loach, PA. (1976) In Handbook of Biochemistry and Molecular Biology, 3rd edn (Fasman,
G.D., ed.), Physical and Chemical Data, Vol. I, pp. 122-130, CRC Press, Boca Raton, FL.
* This is the value for free FAD; FAD bound to a specific flavoprotein (for example succinate dehydrogenase) has a dif-
ferent E" that depends on its protein environments.
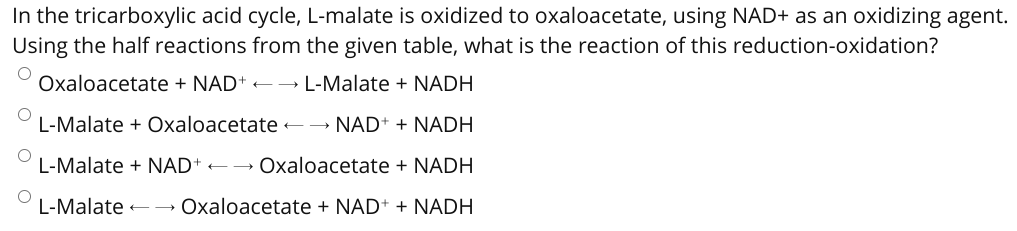
Transcribed Image Text:In the tricarboxylic acid cycle, L-malate is oxidized to oxaloacetate, using NAD+ as an oxidizing agent.
Using the half reactions from the given table, what is the reaction of this reduction-oxidation?
Oxaloacetate + NAD+ +→ L-Malate + NADH
L-Malate + Oxaloacetate -→ NAD+ + NADH
L-Malate + NAD+ +→ Oxaloacetate + NADH
L-Malate -→ Oxaloacetate + NAD+ + NADH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning