Using the maximum work theorem with chemical work The reduction of iron(III) oxide (Fe,O) to pure iron during the first step of steelmaking, 2 Fe2O3(s)4Fe(s) +302(g) is driven by the high-temperature combustion of coke, a purified form of coal: CO2(g) C(s) + O2(g) Suppose at the temperature of a blast furnace the Gibbs free energies of formation AG, of CO, and Fe,O3 are -418. kJ/mol and -849. kJ/mol, respectively. Calculate the maximum 'mass of pure iron that can be produced by the combustion of 350. t of coke. (One metric ton, symbol t, equals 1000 kg.) Round your answer to 2 significant digits. kg x10 ? Check Explanation 2019 McGraw-Hill Education. All Rights Reserved.Terms of Use 1II
Using the maximum work theorem with chemical work The reduction of iron(III) oxide (Fe,O) to pure iron during the first step of steelmaking, 2 Fe2O3(s)4Fe(s) +302(g) is driven by the high-temperature combustion of coke, a purified form of coal: CO2(g) C(s) + O2(g) Suppose at the temperature of a blast furnace the Gibbs free energies of formation AG, of CO, and Fe,O3 are -418. kJ/mol and -849. kJ/mol, respectively. Calculate the maximum 'mass of pure iron that can be produced by the combustion of 350. t of coke. (One metric ton, symbol t, equals 1000 kg.) Round your answer to 2 significant digits. kg x10 ? Check Explanation 2019 McGraw-Hill Education. All Rights Reserved.Terms of Use 1II
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 68E: In a coffee-cup calorimeter, 1.60 g NH4NO3 is mixed with 75.0 g water at an initial temperature of...
Related questions
Question
Calculate the maximum mass of pure iron that can be produced by the concentration of 350 t of coke. Please don't round when doing math
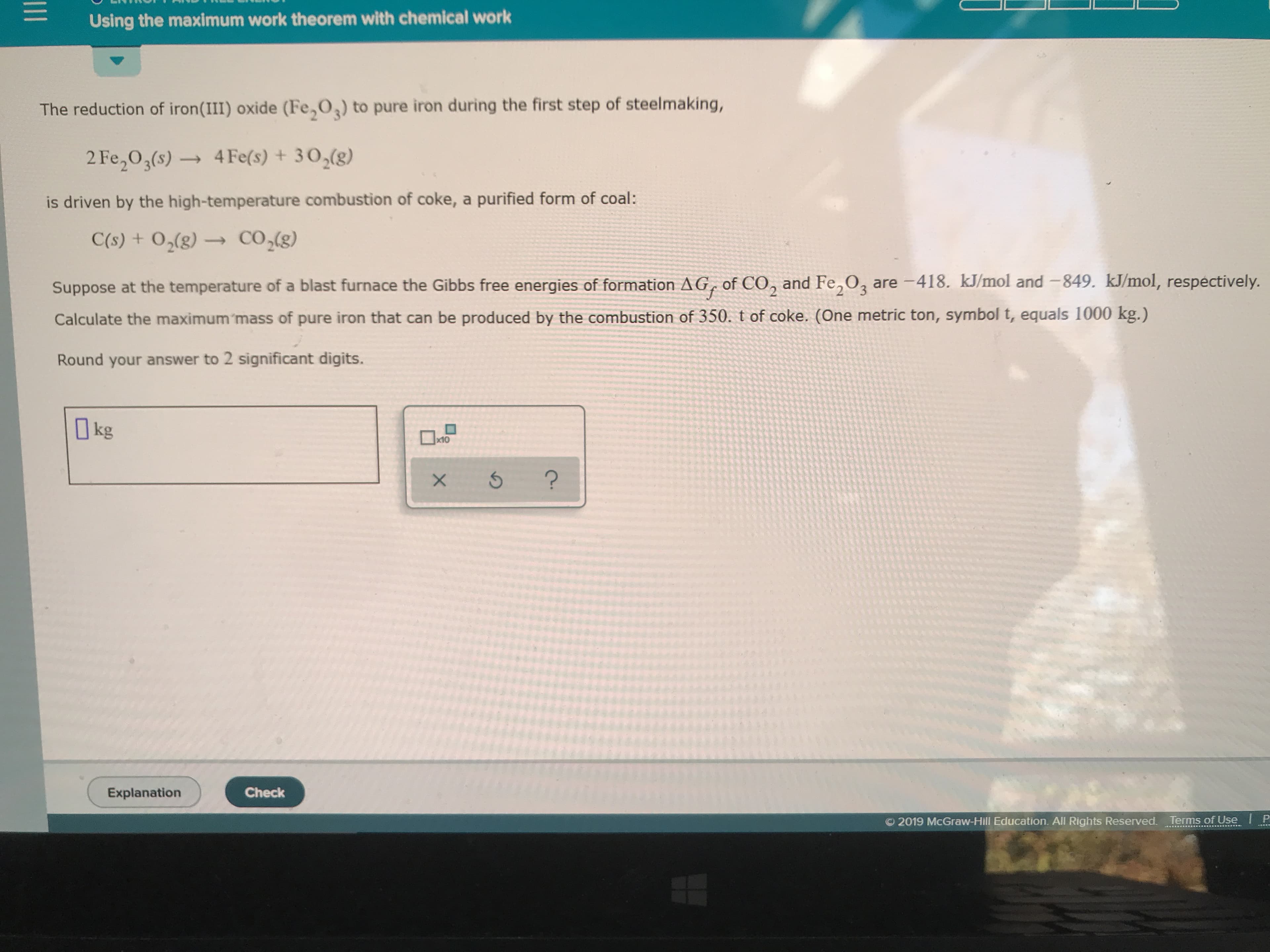
Transcribed Image Text:Using the maximum work theorem with chemical work
The reduction of iron(III) oxide (Fe,O) to pure iron during the first step of steelmaking,
2 Fe2O3(s)4Fe(s) +302(g)
is driven by the high-temperature combustion of coke, a purified form of coal:
CO2(g)
C(s) + O2(g)
Suppose at the temperature of a blast furnace the Gibbs free energies of formation AG, of CO, and Fe,O3 are -418. kJ/mol and -849. kJ/mol, respectively.
Calculate the maximum 'mass of pure iron that can be produced by the combustion of 350. t of coke. (One metric ton, symbol t, equals 1000 kg.)
Round your answer to 2 significant digits.
kg
x10
?
Check
Explanation
2019 McGraw-Hill Education. All Rights Reserved.Terms of Use
1II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning