Vhat specifically is happening at points B>C? What specifically is happening at points D→E? What specifically is happening at points C>B? What specifically is happening at points E>D? At what point does this substance begin to freeze? At what point does this substance begin to melt? At what point does this substance begin to boil? At uhot
Vhat specifically is happening at points B>C? What specifically is happening at points D→E? What specifically is happening at points C>B? What specifically is happening at points E>D? At what point does this substance begin to freeze? At what point does this substance begin to melt? At what point does this substance begin to boil? At uhot
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.73E: Why are steam burns so much worse than water burns even if the H2O is at the same temperature for...
Related questions
Question
100%

Transcribed Image Text:What specifically is happening at points B C?
What specifically is happening at points D>E?
What specifically is happening at points C>B?
What specifically is happening at points E->D?
At what point does this substance begin to freeze?
At what point does this substance begin to melt?
At what point does this substance begin to boil?
At what point does this substance begin to condense?
What is the freezing point of this substance?
What is the melting point of this substance?
What is the boiling point of this substance?
Describe an actual physical situation this graph may be representing (disregard exac
temperatures).
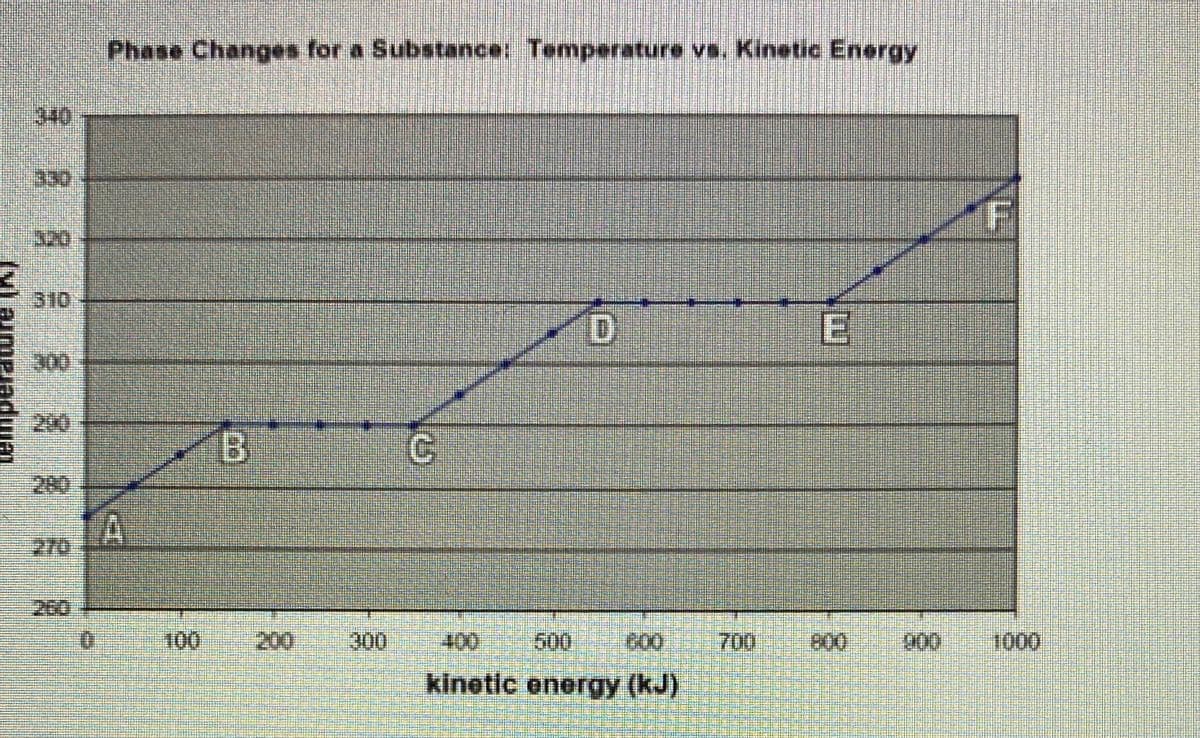
Transcribed Image Text:Phase Changes for a Substance: Temperature vs. Kinetic Energy
340
330
320
310
E
300
B
200
280
A
270
20
100
200
300
400
600
000
700
800
900
1000
kinetic energy (kJ)
(v)arme/adwa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning