We leached 200 g of our soil with 500 mL of NH4CI. The collected solution contained: 320 mg L-1 Ca2+ 168 mg L-1 Mg2+ -1 234 mg L-¹ K+ -1 8 mg L-¹ H+ 144 mg L-1 A13+
We leached 200 g of our soil with 500 mL of NH4CI. The collected solution contained: 320 mg L-1 Ca2+ 168 mg L-1 Mg2+ -1 234 mg L-¹ K+ -1 8 mg L-¹ H+ 144 mg L-1 A13+
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.1QAP
Related questions
Question
The first photo is the data, second photo is the question! thank you!
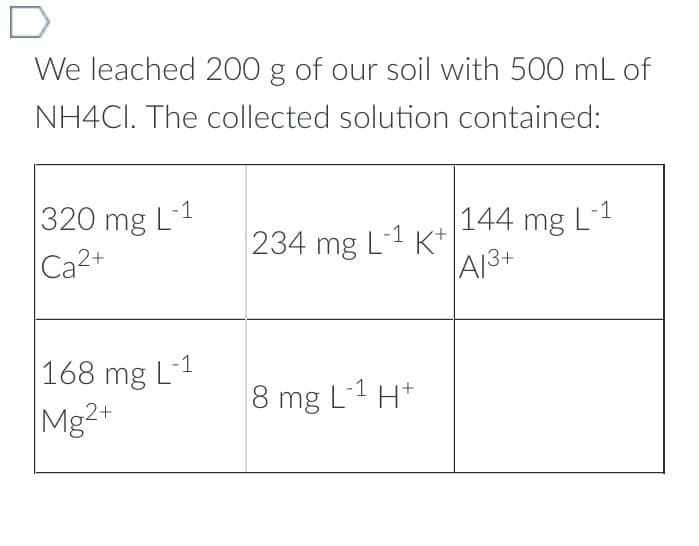
Transcribed Image Text:We leached 200 g of our soil with 500 mL of
NH4CI. The collected solution contained:
320 mg L-1
Ca2+
168 mg L-1
Mg2+
-1
234 mg L-¹ K+
8 mg L-1 H+
144 mg L-1
A13+
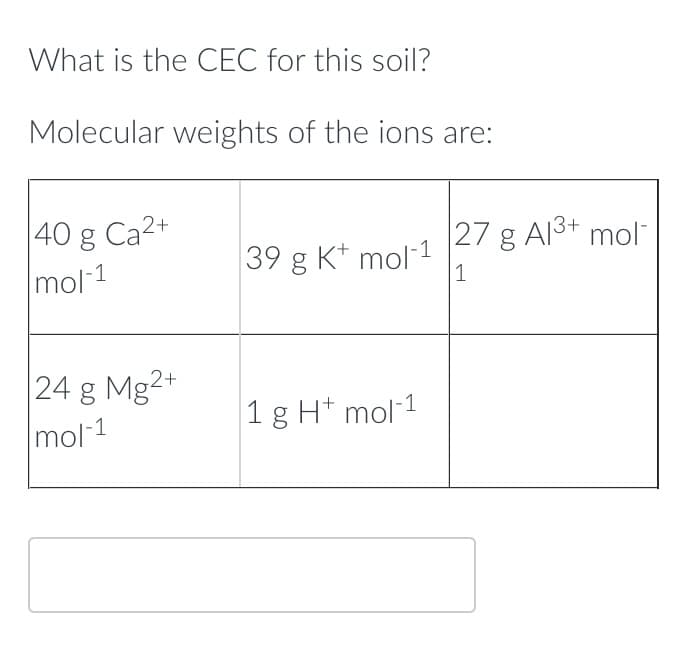
Transcribed Image Text:What is the CEC for this soil?
Molecular weights of the ions are:
40 g Ca²+
mol-1
24 g Mg2+
mol-1
39 g K+ mol-1
1 g Ht mol-1
27 g Al³+ mol
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
thank you!
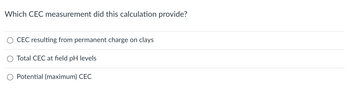
Transcribed Image Text:Which CEC measurement did this calculation provide?
CEC resulting from permanent charge on clays
Total CEC at field pH levels
Potential (maximum) CEC
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning