The reaction P4(g) 2 2 P2(g) is endothermic and begins to occur at moderate temperatures. (a) In which direction do you expect deviations to occur from Boyle's law, P ∞ 1/V (constant T), for gaseous P4? (b) In which direction do you expect deviations to occur from Charles's law, V « T (constant P), for gaseous P4?
The reaction P4(g) 2 2 P2(g) is endothermic and begins to occur at moderate temperatures. (a) In which direction do you expect deviations to occur from Boyle's law, P ∞ 1/V (constant T), for gaseous P4? (b) In which direction do you expect deviations to occur from Charles's law, V « T (constant P), for gaseous P4?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 85QAP: Which of the following quantities can be taken to be independent of temperature? independent of...
Related questions
Question
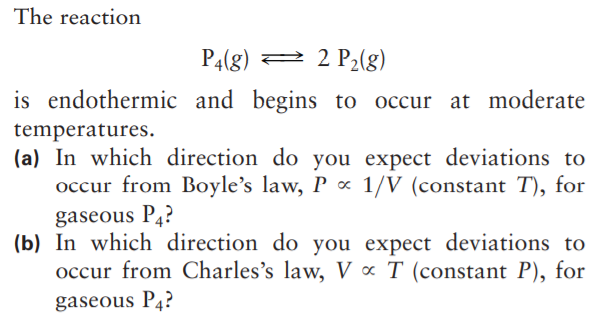
Transcribed Image Text:The reaction
P4(g) 2 2 P2(g)
is endothermic and begins to occur at moderate
temperatures.
(a) In which direction do you expect deviations to
occur from Boyle's law, P ∞ 1/V (constant T), for
gaseous P4?
(b) In which direction do you expect deviations to
occur from Charles's law, V « T (constant P), for
gaseous P4?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning