What is the maximum amount of work that could be done by the following electrochemical cell? Al(s)Al(NO3),(aq)||AGNO;(aq)Ag(s) Hall-Reaction Hal madficen 7 2-4 2.7 1.9 49 Ca eCe LIC RC+ 24 T 1.N SO 2e HSO, HO PhO+ ar Sa 2PASO, 0 M0, + MO,20 7N R L0A 180 1.51 850 -413 Mao, r S M LO A no, ar e O N+ N PhO + s0 146 a0 de- + HO 1.23 MaO+ar2 O Forth + Fe -44 19 LOS Za LIG R20 AaCT M Ma Al -LI AuC 0.9 NO ar+ NO O -223 -37 -217 -271 -276 CO, C0" 0.954 ASt Mrt + 3o -Mg AgeA H 2-Hg Na+ Ca + Ca NO 77 a -HO, Ma Maat -190 a.54 O 1.420 kJ O 949 kJ Stop sh O 237 kJ I1 Proctorio is sharing your screen. O None of these is correct
What is the maximum amount of work that could be done by the following electrochemical cell? Al(s)Al(NO3),(aq)||AGNO;(aq)Ag(s) Hall-Reaction Hal madficen 7 2-4 2.7 1.9 49 Ca eCe LIC RC+ 24 T 1.N SO 2e HSO, HO PhO+ ar Sa 2PASO, 0 M0, + MO,20 7N R L0A 180 1.51 850 -413 Mao, r S M LO A no, ar e O N+ N PhO + s0 146 a0 de- + HO 1.23 MaO+ar2 O Forth + Fe -44 19 LOS Za LIG R20 AaCT M Ma Al -LI AuC 0.9 NO ar+ NO O -223 -37 -217 -271 -276 CO, C0" 0.954 ASt Mrt + 3o -Mg AgeA H 2-Hg Na+ Ca + Ca NO 77 a -HO, Ma Maat -190 a.54 O 1.420 kJ O 949 kJ Stop sh O 237 kJ I1 Proctorio is sharing your screen. O None of these is correct
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.128QP: a Calculate G for the following cell reaction: Tl(s)Tl+(aq)Pb2+(aq)Pb(s) The Gf for Tl+(aq) is 32.4...
Related questions
Question
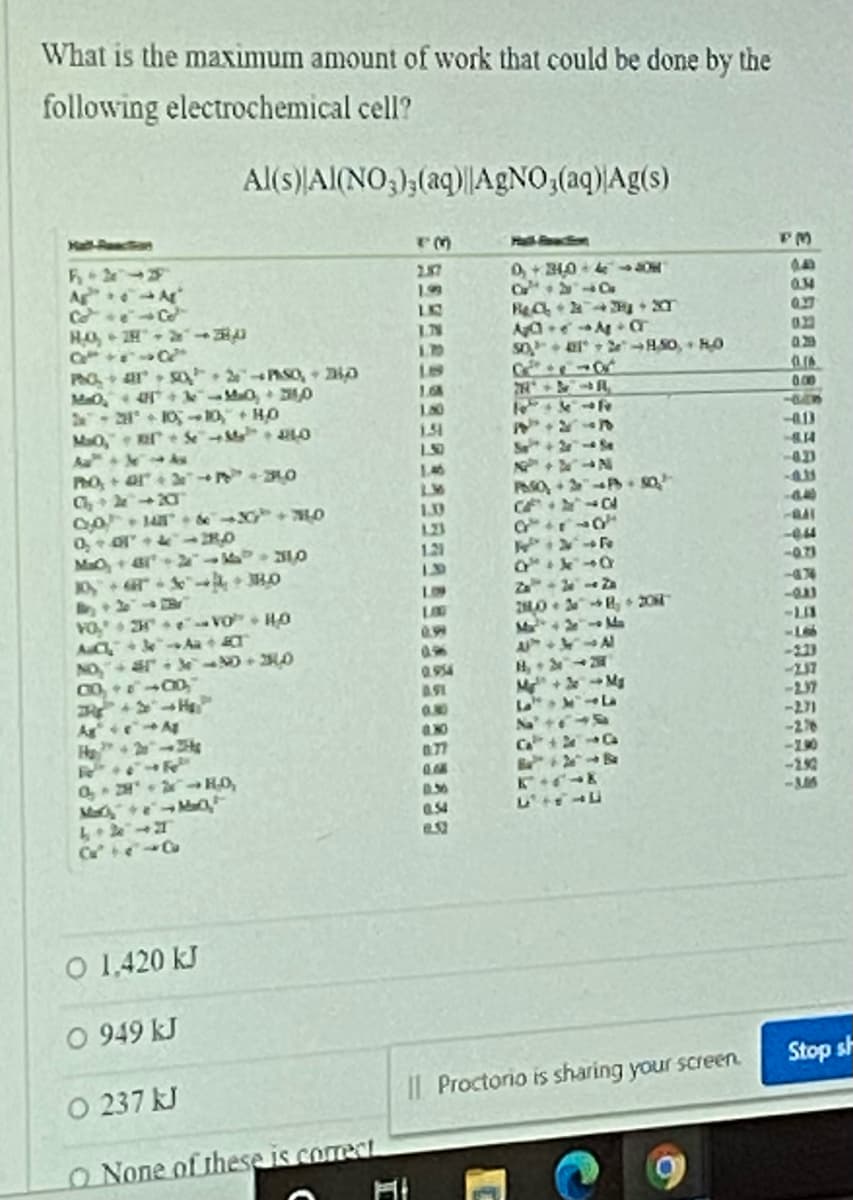
Transcribed Image Text:What is the maximum amount of work that could be done by the
following electrochemical cell?
Al(s) Al(NO,),(aq)||A£NO,(aq)|Ag(s)
Hall-Raacfion
2.87
A A
Co Ce
HO, 2H
40
19
LKB
I.N
1.10
50
401t > e So, HO
PhO+ ar So 2PASO, 0
+ Mao, 0
10A
180
00
2 1O10, HO
Mo, SM O
Au As
Po,+ ar O
1.51
-413
S+2 Se
146
423
Ph0
0o de +HO
1.30
123
1.21
MaC+ar ILO
10 " HO
444
LOS
VO, e Vơ H0
AuC e
NO
LOG
-LII
Ma
A
H,
CT
0.99
Ma
-Le
-223
0.954
ASI
Mg
0.00
AgeA
H+2-s
-271
-2개
-190
-190
-M6
Na+
a77
Ca+ Ca
Ma eMaO
0.54
O 1.420 kJ
O 949 kJ
Stop sh
O 237 kJ
Il Proctorio is sharing your screen.
O None of these is correct
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning