2. The following cell is set up: 2. The following cell is set up: Porous Barrier 0.01M Pb(NO32 pH - 10.0 0.1M KMN04 Pt Pb a) What are the reactions taking place at the electrodes? b) What will the cell voltage be? c) What is the maximum amount of work you can get from this cell (per mole of electrons)?
2. The following cell is set up: 2. The following cell is set up: Porous Barrier 0.01M Pb(NO32 pH - 10.0 0.1M KMN04 Pt Pb a) What are the reactions taking place at the electrodes? b) What will the cell voltage be? c) What is the maximum amount of work you can get from this cell (per mole of electrons)?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 67E: A galvanic cell is based on the following half-reactions at 25C: Ag++eAg H2O2+2H++2e2H2O Predict...
Related questions
Question
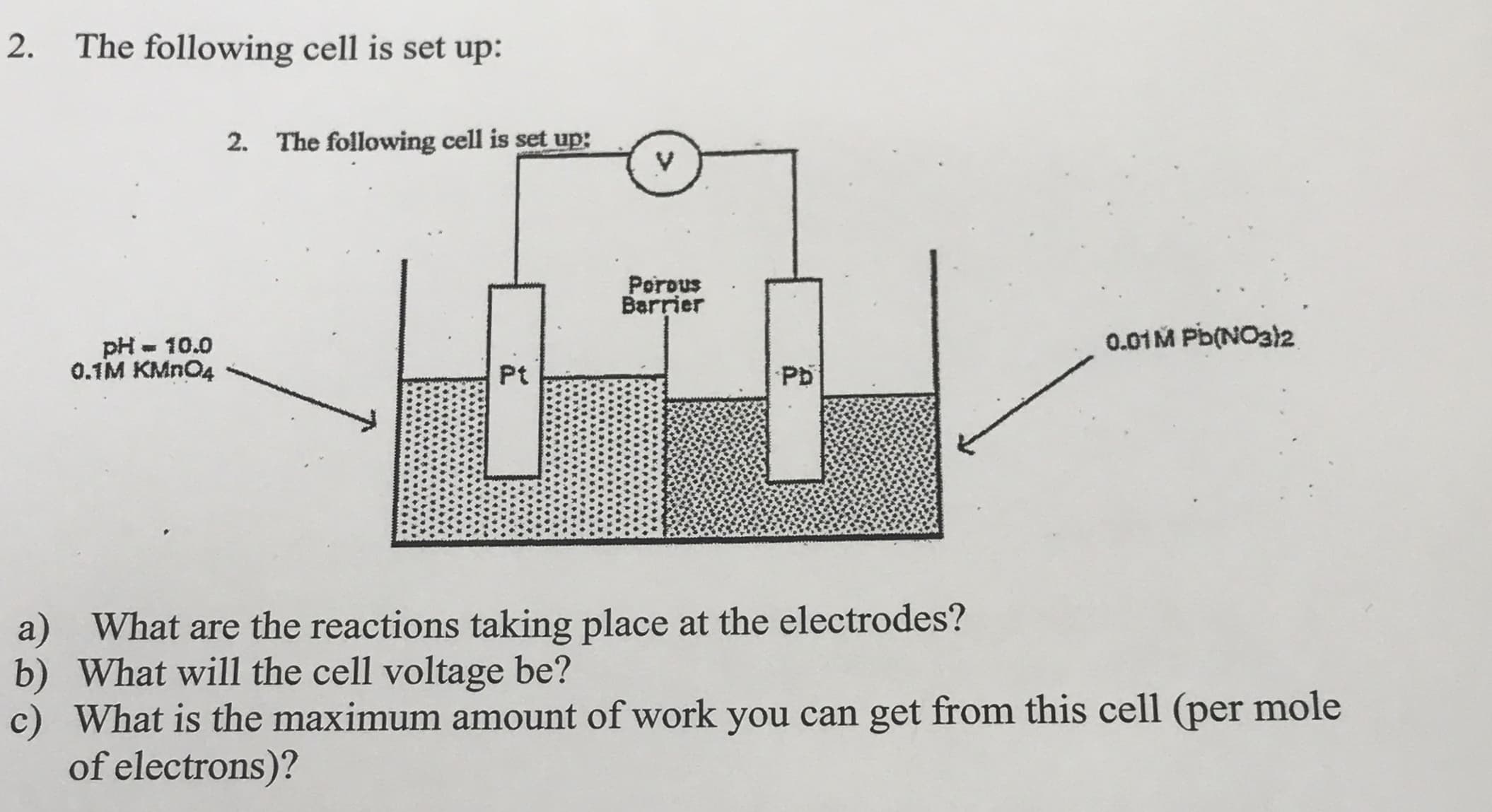
Transcribed Image Text:2.
The following cell is set up:
2. The following cell is set up:
Porous
Barrier
0.01M Pb(NO32
pH - 10.0
0.1M KMN04
Pt
Pb
a) What are the reactions taking place at the electrodes?
b) What will the cell voltage be?
c) What is the maximum amount of work you can get from this cell (per mole
of electrons)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning