Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter6: The Periodic Table And Periodic Law
Section: Chapter Questions
Problem 66A
Related questions
Question
Answer question 18
Transcribed Image Text:ons Help Accessibility
Last edit wa 4 hours ago
pen Sans
BIU
A
E = E E 1E E - E
1 1
12
2 1 3 4
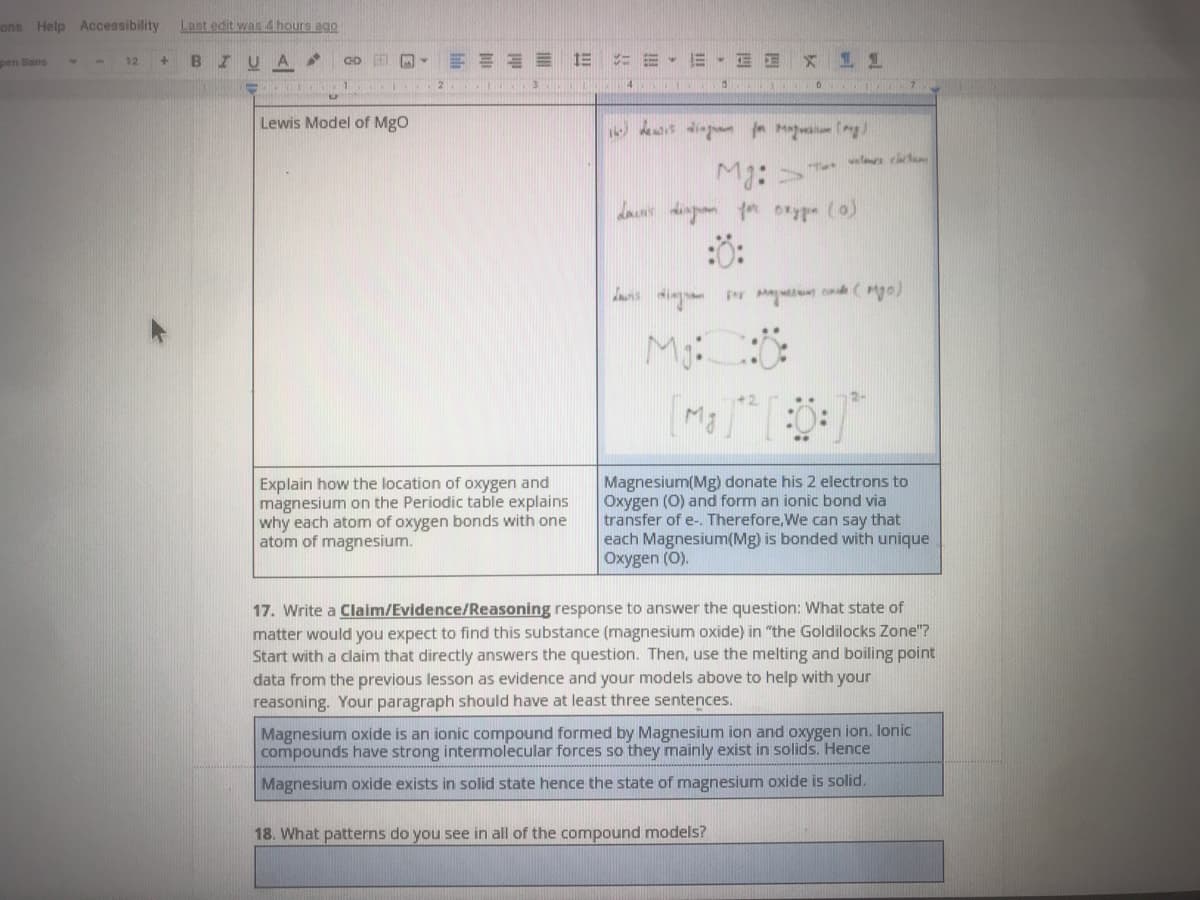
Lewis Model of Mgo
T wal t
Mg:
da dingn o oxyp (o)
s ing Pr Ma (Mya)
Explain how the location of oxygen and
magnesium on the Periodic table explains
why each atom of oxygen bonds with one
atom of magnesium.
Magnesium(Mg) donate his 2 electrons to
Oxygen (0) and form an ionic bond via
transfer of e-. Therefore,We can say that
each Magnesium(Mg) is bonded with unique
Oxygen (O).
17. Write a Claim/Evidence/Reasoning response to answer the question: What state of
matter would you expect to find this substance (magnesium oxide) in "the Goldilocks Zone"?
Start with a claim that directly answers the question. Then, use the melting and boiling point
data from the previous lesson as evidence and your models above to help with your
reasoning. Your paragraph should have at least three sentences.
Magnesium oxide is an ionic compound formed by Magnesium ion and oxygen ion. lonic
compounds have strong intermolecular forces so they mainly exist in solids. Hence
Magnesium oxide exists in solid state hence the state of magnesium oxide is solid.
18. What patterns do you see in all of the compound models?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning