Question is attached

The amide functional group is denoted as R-CO-NH2, where R can be alky, aryl, or hydrogen. When the amide constitutes the ring, it is termed as the lactam. The most common method to synthesis the amide is the reaction of the acid chloride with an amine. The amide linkage can be cleaved by treating with the base to furnish the acid and amine. The added base should be nucleophilic enough to react with the carbon of the carbonyl group.
Reaction:
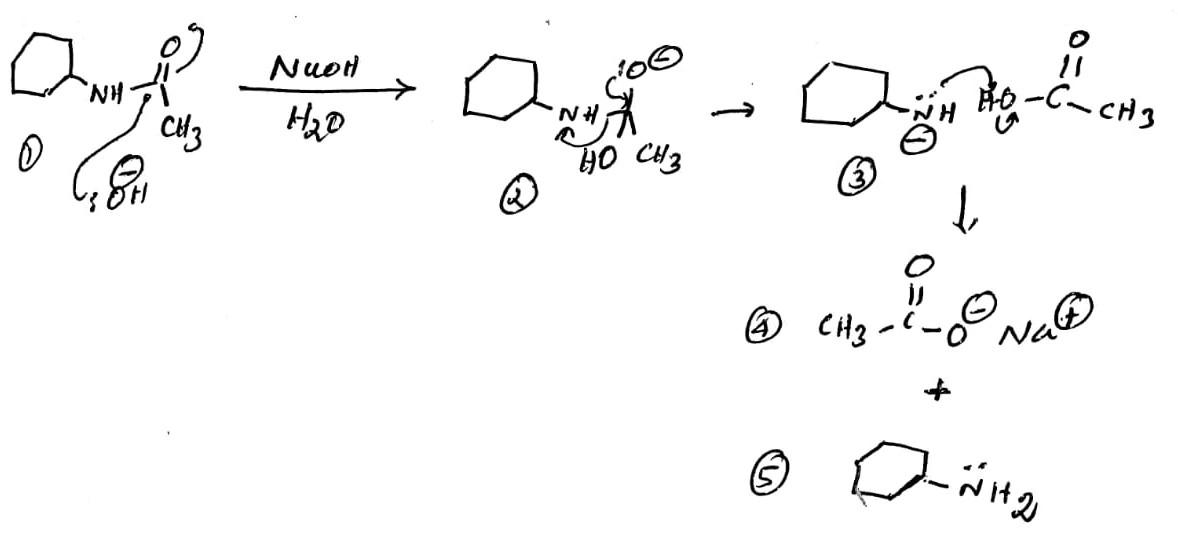
Explanation:
The nucleophilic addition of the hydroxide ion to the amide 1 produces the oxygen anion 2. The shift of the electrons from the oxygen towards the carbon furnishes the amine anion and the acetic acid (3). The anion of amine abstracts the proton from the acetic acid to furnish the sodium acetate 4 and the cyclohexylamine 5.
Step by step
Solved in 3 steps with 1 images






