When 12.0 mL of a 4.66x10-4 M lead acetate solution is combined with 12.0 mL of a 2.33×104 M ammonium phosphate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to When 18.0 mL of a 2.87×10-4 M silver acetate solution is combined with 12.0 mL of a 9.53×104M sodium hydroxide solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to When 25.0 mL of a 7.74x10* M potassium hydroxide solution is combined with 18.0 mL of a 8.24×10M aluminum sulfate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to
When 12.0 mL of a 4.66x10-4 M lead acetate solution is combined with 12.0 mL of a 2.33×104 M ammonium phosphate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to When 18.0 mL of a 2.87×10-4 M silver acetate solution is combined with 12.0 mL of a 9.53×104M sodium hydroxide solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to When 25.0 mL of a 7.74x10* M potassium hydroxide solution is combined with 18.0 mL of a 8.24×10M aluminum sulfate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 70AP
Related questions
Question
100%
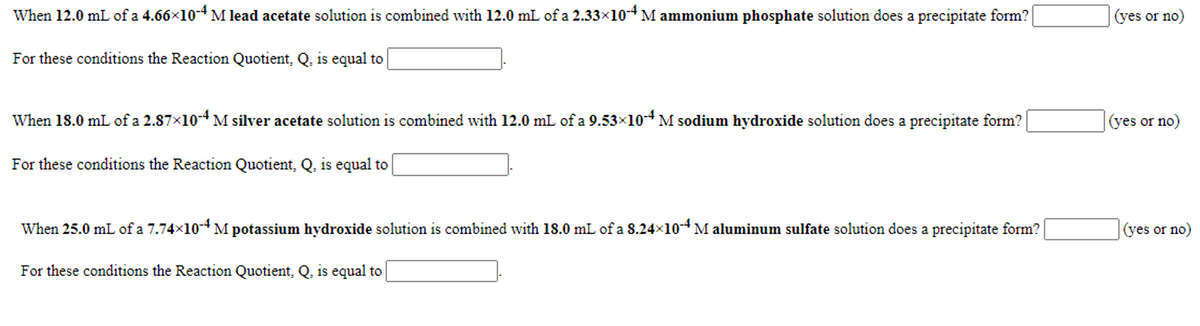
Transcribed Image Text:When 12.0 mL of a 4.66x10-4 M lead acetate solution is combined with 12.0 mL of a 2.33×10-4 M ammonium phosphate solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to
When 18.0 mL of a 2.87x10-4 M silver acetate solution is combined with 12.0 mL of a 9.53x10-4 M sodium hydroxide solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to
When 25.0 mL of a 7.74x10-* M potassium hydroxide solution is combined with 18.0 mL of a 8.24x10*M aluminum sulfate solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
