When 2-methylnaphthalene undergoes an irreversible electrophilic aromatic substitution, the electrophile predominantly attaches to the 1 position instead of the 3 position. This suggests that the arenium ion that is formed from attachment of the electrophile to the 1 position is more stable than the arenium ion formed from attachment of the electrophile to the 3 position. Explain why this is so. Hint: Simple resonance theory can explain why. CH3 CH3 CH3 + 2-Methylnaphthalene Major product
When 2-methylnaphthalene undergoes an irreversible electrophilic aromatic substitution, the electrophile predominantly attaches to the 1 position instead of the 3 position. This suggests that the arenium ion that is formed from attachment of the electrophile to the 1 position is more stable than the arenium ion formed from attachment of the electrophile to the 3 position. Explain why this is so. Hint: Simple resonance theory can explain why. CH3 CH3 CH3 + 2-Methylnaphthalene Major product
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter19: Eas: Electrophilic Aromatic Substitution
Section: Chapter Questions
Problem 12E
Related questions
Question
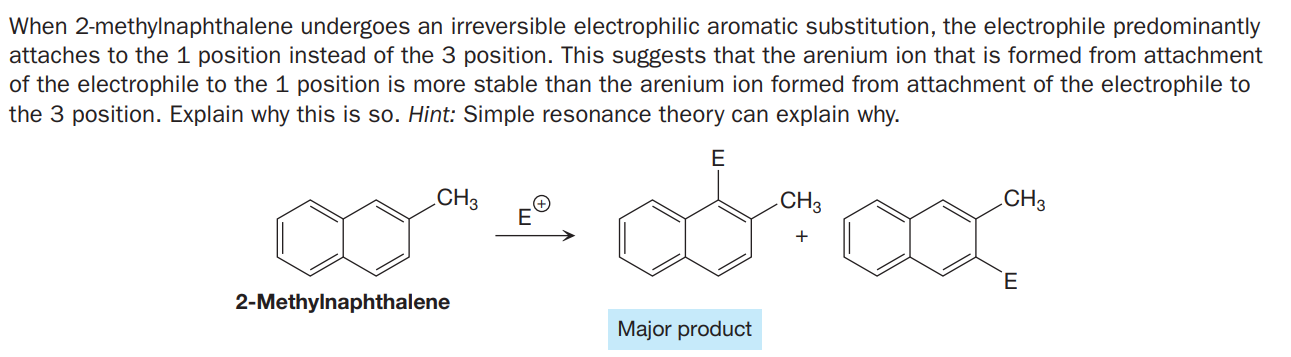
Transcribed Image Text:When 2-methylnaphthalene undergoes an irreversible electrophilic aromatic substitution, the electrophile predominantly
attaches to the 1 position instead of the 3 position. This suggests that the arenium ion that is formed from attachment
of the electrophile to the 1 position is more stable than the arenium ion formed from attachment of the electrophile to
the 3 position. Explain why this is so. Hint: Simple resonance theory can explain why.
CH3
CH3
CH3
+
2-Methylnaphthalene
Major product
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning