When a certain weak-field ligand forms an octahedral complex with Coʻ 2+ cation, the energies of the valence d orbitals on the cobalt atom are split according to this electron box diagram: energy in kJ/mol -300 -350 只只 -400 alo -450 -y Ar -500 -550 -600 -650 -700 ху yz -750 Using this diagram, answer the following questions. How many unpaired d electron spins does the cobalt atom have? paramagnetic Is the complex paramagnetic or diamagnetic? diamagnetic O blue or green
When a certain weak-field ligand forms an octahedral complex with Coʻ 2+ cation, the energies of the valence d orbitals on the cobalt atom are split according to this electron box diagram: energy in kJ/mol -300 -350 只只 -400 alo -450 -y Ar -500 -550 -600 -650 -700 ху yz -750 Using this diagram, answer the following questions. How many unpaired d electron spins does the cobalt atom have? paramagnetic Is the complex paramagnetic or diamagnetic? diamagnetic O blue or green
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter8: Bonding In Transition Metal Compounds And Coordination Complexes
Section: Chapter Questions
Problem 33P
Related questions
Question
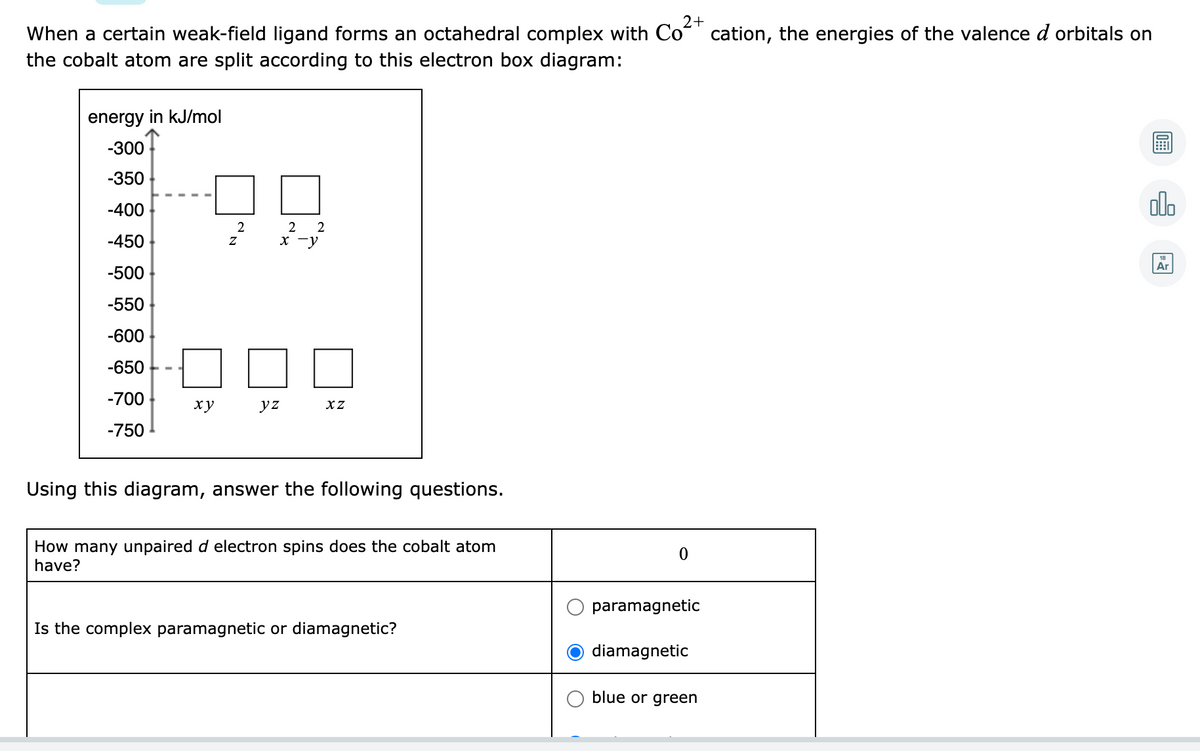
Transcribed Image Text:2+
When a certain weak-field ligand forms an octahedral complex with Co“' cation, the energies of the valence d orbitals on
the cobalt atom are split according to this electron box diagram:
energy in kJ/mol
-300
-350
-400
olo
2
2 2
-450
x -y
Ar
-500
-550
-600
-650
-700
ху
yz
XZ
-750
Using this diagram, answer the following questions.
How many unpaired d electron spins does the cobalt atom
have?
paramagnetic
Is the complex paramagnetic or diamagnetic?
diamagnetic
blue or green
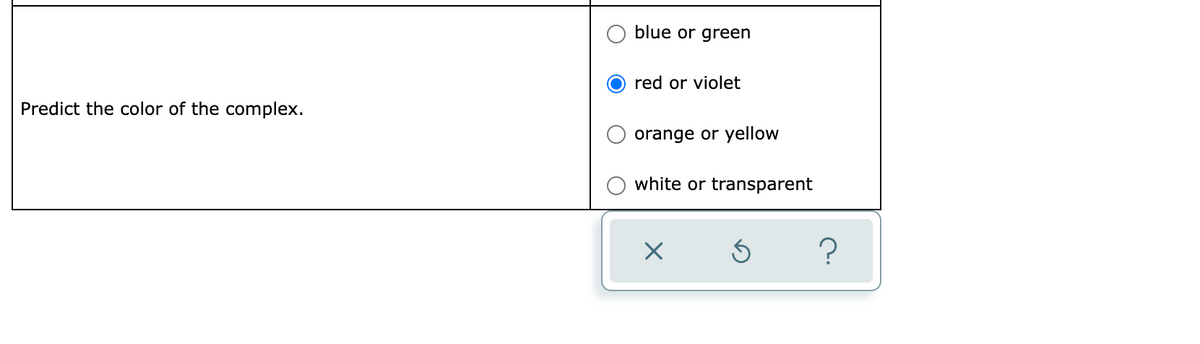
Transcribed Image Text:blue or green
red or violet
Predict the color of the complex.
orange or yellow
white or transparent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning