When an excess of sodium hydroxide is added to an aque- ous solution of ammonium chloride, gaseous ammonia is produced: NaOH(aq) + NH,Cl(aq) - NaCl(aq) + NH;(g) + H,O(€) Suppose 3.68 g ammonium chloride reacts in this way at 30°C and a total pressure of 0.9884 atm. At this tempera- ture, the vapor pressure of water is 0.0419 atm. Calculate the volume of ammonia saturated with water vapor that will be produced under these conditions, assuming no leaks or other losses of gas.
When an excess of sodium hydroxide is added to an aque- ous solution of ammonium chloride, gaseous ammonia is produced: NaOH(aq) + NH,Cl(aq) - NaCl(aq) + NH;(g) + H,O(€) Suppose 3.68 g ammonium chloride reacts in this way at 30°C and a total pressure of 0.9884 atm. At this tempera- ture, the vapor pressure of water is 0.0419 atm. Calculate the volume of ammonia saturated with water vapor that will be produced under these conditions, assuming no leaks or other losses of gas.
Chapter5: Gases
Section: Chapter Questions
Problem 74E: Urea (H2NCONH2) is used extensively as a nitrogen source in fertilizers. It is produced commercially...
Related questions
Question
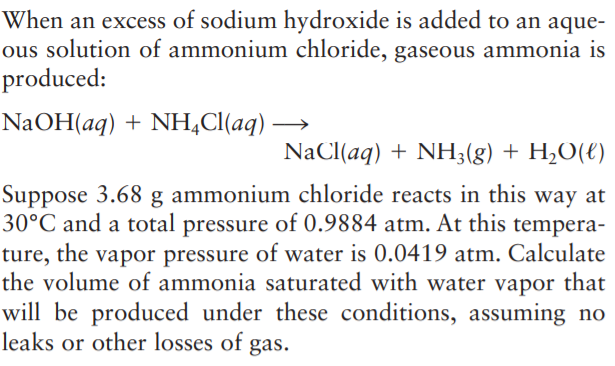
Transcribed Image Text:When an excess of sodium hydroxide is added to an aque-
ous solution of ammonium chloride, gaseous ammonia is
produced:
NaOH(aq) + NH,Cl(aq) -
NaCl(aq) + NH;(g) + H,O(€)
Suppose 3.68 g ammonium chloride reacts in this way at
30°C and a total pressure of 0.9884 atm. At this tempera-
ture, the vapor pressure of water is 0.0419 atm. Calculate
the volume of ammonia saturated with water vapor that
will be produced under these conditions, assuming no
leaks or other losses of gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning