The average kinetic energy of the molecules in a gas sample depends only on the temperature, T. However, given the same kinetic energies, a lighter molecule will move faster than a heavier molecule, as shown in the equation for rms speed 3RT rms speed = where R = 8.314 J/ (mol·K) and M is molar mass in kilograms per mole. Note that a joule is the same as a kilogram-meter squared per second squared (kg-m?/s²). What is the rms speed of F, molecules at 485 K?
The average kinetic energy of the molecules in a gas sample depends only on the temperature, T. However, given the same kinetic energies, a lighter molecule will move faster than a heavier molecule, as shown in the equation for rms speed 3RT rms speed = where R = 8.314 J/ (mol·K) and M is molar mass in kilograms per mole. Note that a joule is the same as a kilogram-meter squared per second squared (kg-m?/s²). What is the rms speed of F, molecules at 485 K?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 104QRT
Related questions
Question
I need help with this chemistry homework thanks!
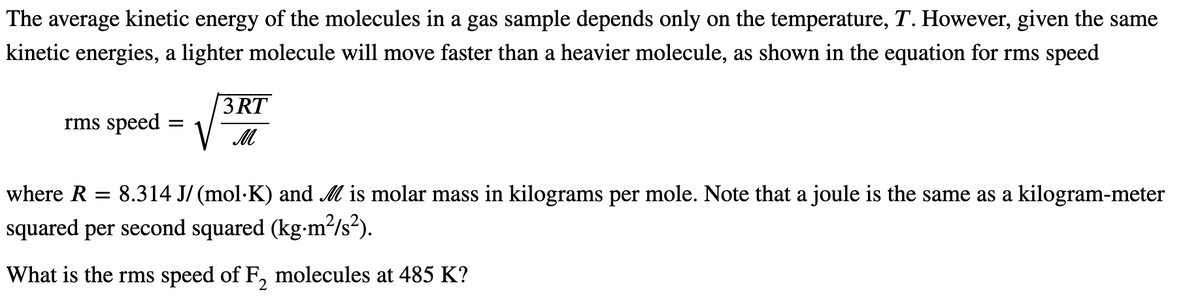
Transcribed Image Text:The average kinetic energy of the molecules in a gas sample depends only on the temperature, T. However, given the same
kinetic energies, a lighter molecule will move faster than a heavier molecule, as shown in the equation for rms speed
3 RT
rms speed
where R = 8.314 J/ (mol·K) and M is molar mass in kilograms per mole. Note that a joule is the same as a kilogram-meter
squared per second squared (kg-m²/s²).
What is the rms speed of F, molecules at 485 K?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning