When heated, KCIO, decomposes into KCl and O,. 2 KCIO, → 2 KCI + 3 O, - If this reaction produced 29.4 g KCl, how many grams of O, were produced?
When heated, KCIO, decomposes into KCl and O,. 2 KCIO, → 2 KCI + 3 O, - If this reaction produced 29.4 g KCl, how many grams of O, were produced?
Chapter4: Molecules, Compounds, And Chemical Reactions
Section: Chapter Questions
Problem 54E: Billions of pounds of urea, CO(NH2)2, are produced annually for use as a fertilizer. The principal...
Related questions
Question
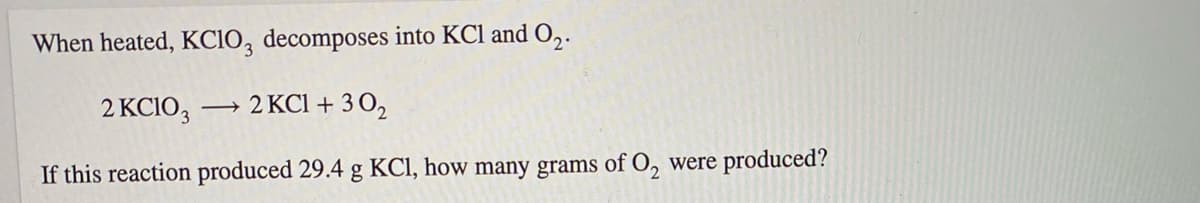
Transcribed Image Text:When heated, KCIO, decomposes into KCl and ,.
2 KCIO,
→ 2 KCI + 3 0,
If this reaction produced 29.4 g KCl, how many grams of O, were produced?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning