Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Transcribed Image Text:earpod - 7 Reaction Rates (1) x
Rosenthal_7th Period 361989
E Percent Yield Practice - Abby
In an ex
ment/d/1PUWBJG1E-gz91BfKaDwbXXC80c3jxjE9fJzg9vbM7eQ/edit?userid=atanner30@studentwps.org18067728s
oby Tanner D O
at Tools Add-ons Help
Last edit was seconds ago
nal text
Calibri
BIUA
11
EI
I 1 I 2 I 3 L 4 I
6 II7
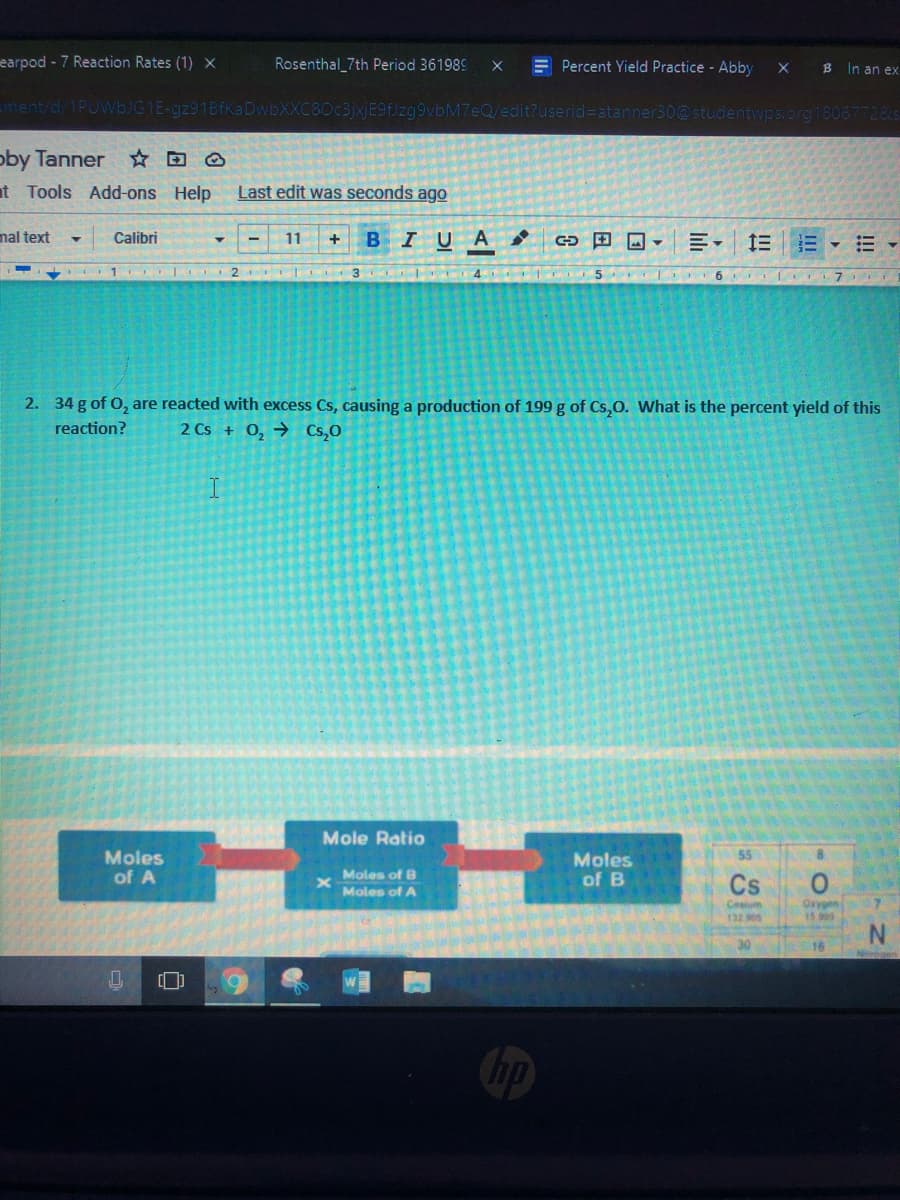
2. 34 g of O, are reacted with excess Cs, causing a production of 199 g of Cs,o. What is the percent yield of this
reaction?
2 Cs + 0, → Cs,0
Mole Ratio
Moles
of A
55
Moles of B
Moles of A
Moles
of B
Cs
Cesim
132 905
Oxygen
13 09
16
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



