When hydroxide is used as the base to carry out an E2 reaction on a vinylic halide, the reaction usually needs to be heated significantly. As shown below, such a reaction involving the E isomer typically requires much higher temperatures. Why is this so? Z isomer E isomer Br КОН КОН 70 °C 200-230 °C Br Br 70% 67%
When hydroxide is used as the base to carry out an E2 reaction on a vinylic halide, the reaction usually needs to be heated significantly. As shown below, such a reaction involving the E isomer typically requires much higher temperatures. Why is this so? Z isomer E isomer Br КОН КОН 70 °C 200-230 °C Br Br 70% 67%
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 74AP
Related questions
Question
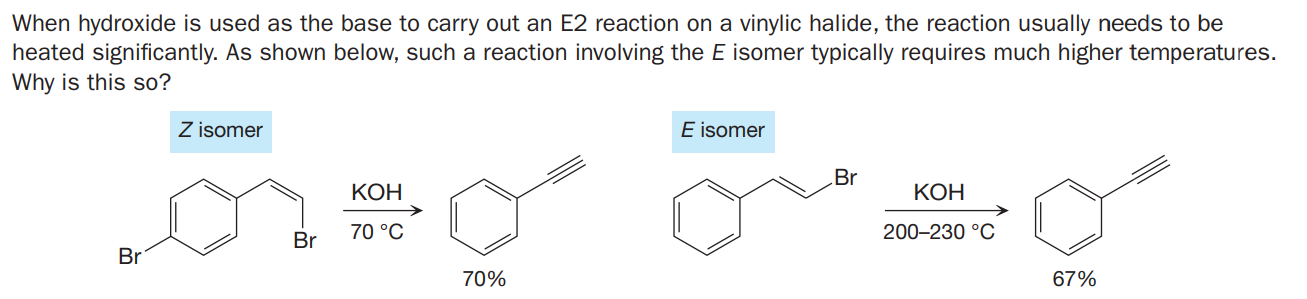
Transcribed Image Text:When hydroxide is used as the base to carry out an E2 reaction on a vinylic halide, the reaction usually needs to be
heated significantly. As shown below, such a reaction involving the E isomer typically requires much higher temperatures.
Why is this so?
Z isomer
E isomer
Br
КОН
КОН
70 °C
200-230 °C
Br
Br
70%
67%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning