When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? CIO₂ + Cr³+ ClO3 + Cr²+ Water appears in the balanced equation as a How many electrons are transferred in this reaction? (reactant, product, neither) with a coefficient of (Enter 0 for neither.)
When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? CIO₂ + Cr³+ ClO3 + Cr²+ Water appears in the balanced equation as a How many electrons are transferred in this reaction? (reactant, product, neither) with a coefficient of (Enter 0 for neither.)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter19: Transition Metals And Coordination Chemistry
Section: Chapter Questions
Problem 17E: Predict the products of each of the following reactions. (Note: In addition to using the information...
Related questions
Question
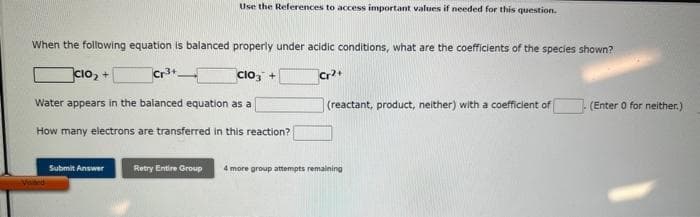
Transcribed Image Text:When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?
CIO₂ +
Cr³+
ClO3 +
Cr2+
Water appears in the balanced equation as a
How many electrons are transferred in this reaction?
Visited
Use the References to access important values if needed for this question.
Submit Answer
(reactant, product, neither) with a coefficient of
Retry Entire Group 4 more group attempts remaining
(Enter 0 for neither.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning