When two atoms form a single covalent bond, two orbitals (one from each atom) overlap such that the electron pair can be in both orbitals simultaneously. Double and triple bonds involve the sharing of multiple electron pairs. Each additional electron pair requires the overlap of another set of orbitals. A set of overlapping orbitals is called a o bond (sigma bond) if the overlap is head-on and a r bond (pi bond) if the overlap is sideways. Part A How many o and T bonds are present in a molecule of cumulene? (Figure 1) Enter the number of o bonds followed by the number of T bonds separated by a comma. • View Available Hint(s) Figure 1 of 1 H `C=c=C=C `H
When two atoms form a single covalent bond, two orbitals (one from each atom) overlap such that the electron pair can be in both orbitals simultaneously. Double and triple bonds involve the sharing of multiple electron pairs. Each additional electron pair requires the overlap of another set of orbitals. A set of overlapping orbitals is called a o bond (sigma bond) if the overlap is head-on and a r bond (pi bond) if the overlap is sideways. Part A How many o and T bonds are present in a molecule of cumulene? (Figure 1) Enter the number of o bonds followed by the number of T bonds separated by a comma. • View Available Hint(s) Figure 1 of 1 H `C=c=C=C `H
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 67P
Related questions
Question
Please answer question 8 part A and B
Transcribed Image Text:Part B
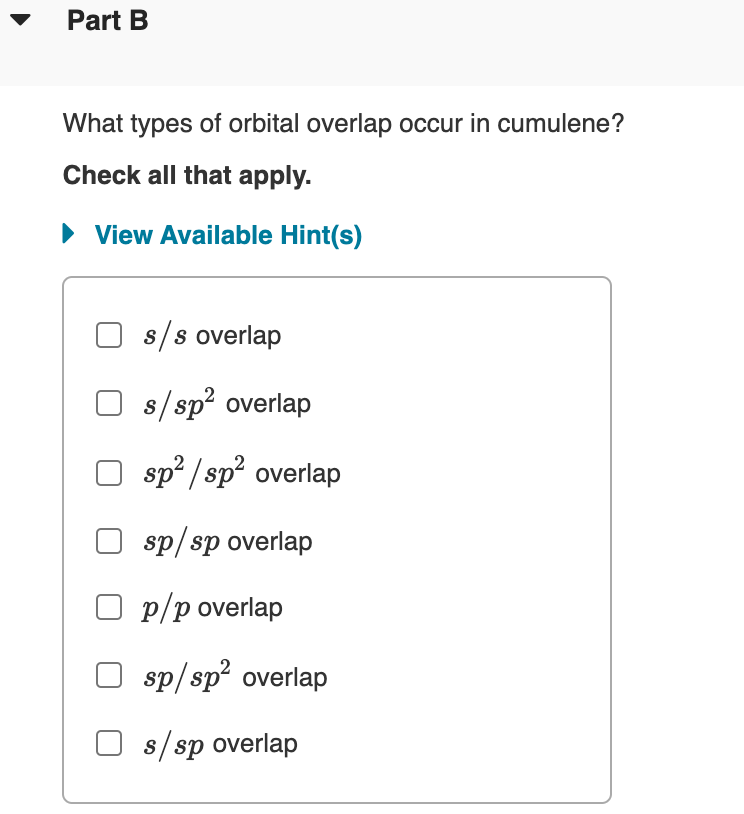
What types of orbital overlap occur in cumulene?
Check all that apply.
• View Available Hint(s)
s/s overlap
s/sp overlap
sp² / sp? overlap
sp/sp overlap
O p/p overlap
sp/ sp overlap
O s/sp overlap
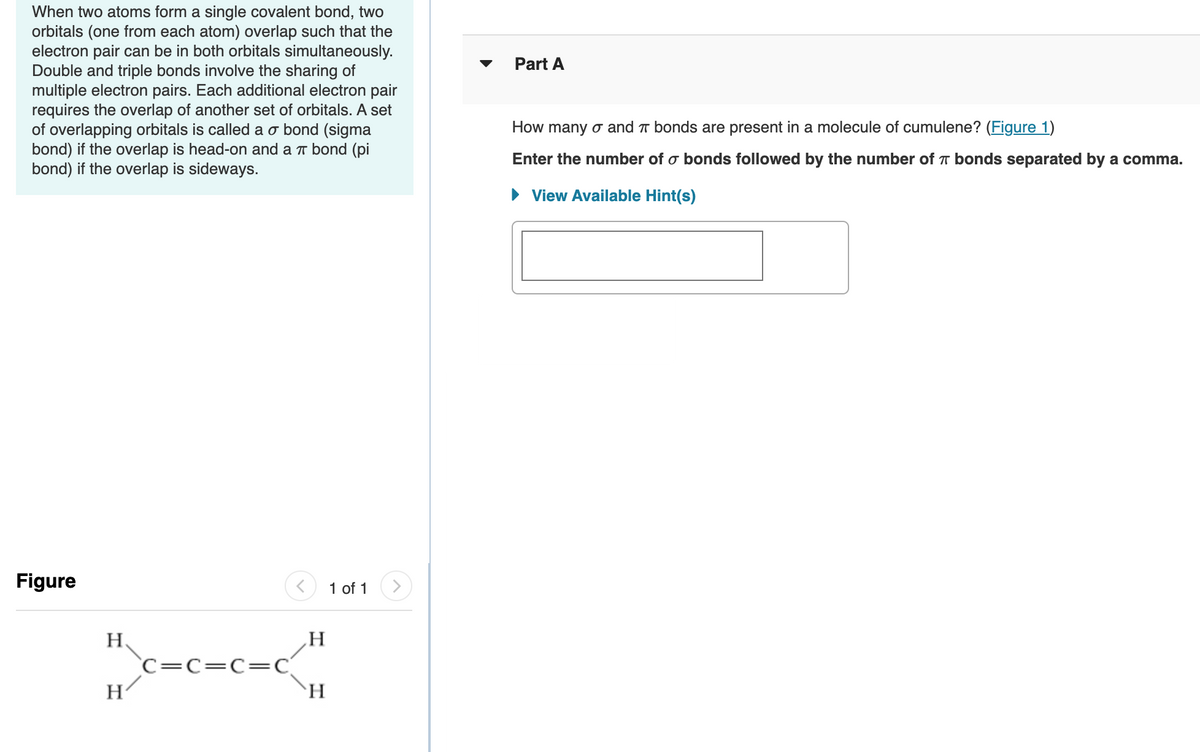
Transcribed Image Text:When two atoms form a single covalent bond, two
orbitals (one from each atom) overlap such that the
electron pair can be in both orbitals simultaneously.
Double and triple bonds involve the sharing of
multiple electron pairs. Each additional electron pair
requires the overlap of another set of orbitals. A set
of overlapping orbitals is called a o bond (sigma
bond) if the overlap is head-on and a T bond (pi
bond) if the overlap is sideways.
Part A
How many o and T bonds are present in a molecule of cumulene? (Figure 1)
Enter the number of o bonds followed by the number of T bonds separated by a comma.
• View Available Hint(s)
Figure
1 of 1
H.
C=c=C=C
H/
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning