Which change to an equilibrium mixture of this reaction results in the formation of more H,S? O an increase in the amount of NH, HS in the reaction vessel O an increase in temperature O a decrease in the volume of the reaction vessel (at constant temperature) all of the ahouo
Which change to an equilibrium mixture of this reaction results in the formation of more H,S? O an increase in the amount of NH, HS in the reaction vessel O an increase in temperature O a decrease in the volume of the reaction vessel (at constant temperature) all of the ahouo
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 3P
Related questions
Question
Transcribed Image Text:apter 15 - Chemical Equilibrium
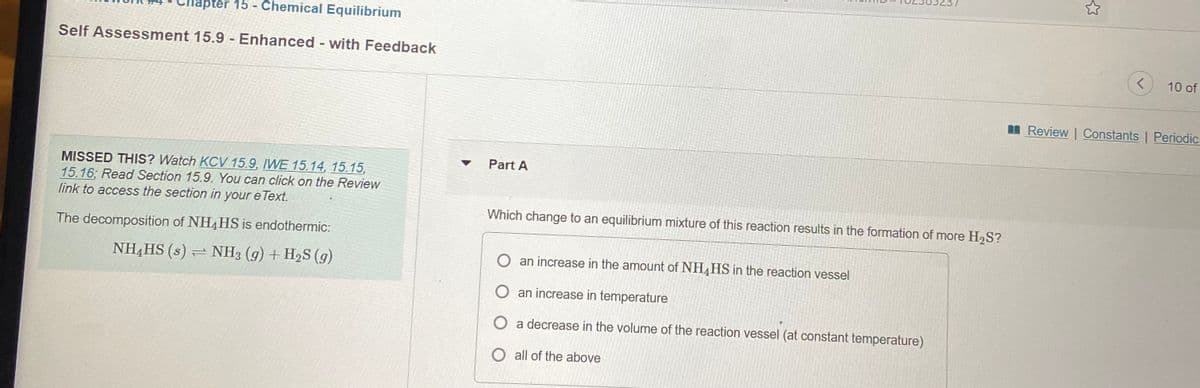
Self Assessment 15.9 - Enhanced - with Feedback
10 of
n Review | Constants | Periodic
Part A
MISSED THIS? Watch KCV15.9, IWE 15.14, 15.15,
15.16; Read Section 15.9. You can click on the Review
link to access the section in your e Text.
Which change to an equilibrium mixture of this reaction results in the formation of more H2S?
The decomposition of NH4HS is endothermic:
NH,HS (s) = NH3 (g) + H2S (g)
O an increase in the amount of NH4HS in the reaction vessel
O an increase in temperature
O a decrease in the volume of the reaction vessel (at constant temperature)
O all of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

