Which of the following combinations of chemicals makes a buffer? Mark all that apply. (Identifying solutions as buffers on a quiz or exam is a critical skill. For this chapter, many problems ask you to find the pH of a solution. The challenge is classifying the solution appropriately, and then selecting the right approach to finding its pH.) O solution that is 0.1 M each in hydrochloric acid and sodium chloride O solution made by combining 25 mL of 0.1 M acetic acid with 12.5 mL of 0.1 M sodium hydroxide O solution that is 0.1 M each in ammonia and ammonium chloride O roughly equimolar mixture of acetic acid and sodium acetate
Which of the following combinations of chemicals makes a buffer? Mark all that apply. (Identifying solutions as buffers on a quiz or exam is a critical skill. For this chapter, many problems ask you to find the pH of a solution. The challenge is classifying the solution appropriately, and then selecting the right approach to finding its pH.) O solution that is 0.1 M each in hydrochloric acid and sodium chloride O solution made by combining 25 mL of 0.1 M acetic acid with 12.5 mL of 0.1 M sodium hydroxide O solution that is 0.1 M each in ammonia and ammonium chloride O roughly equimolar mixture of acetic acid and sodium acetate
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.107E
Related questions
Question
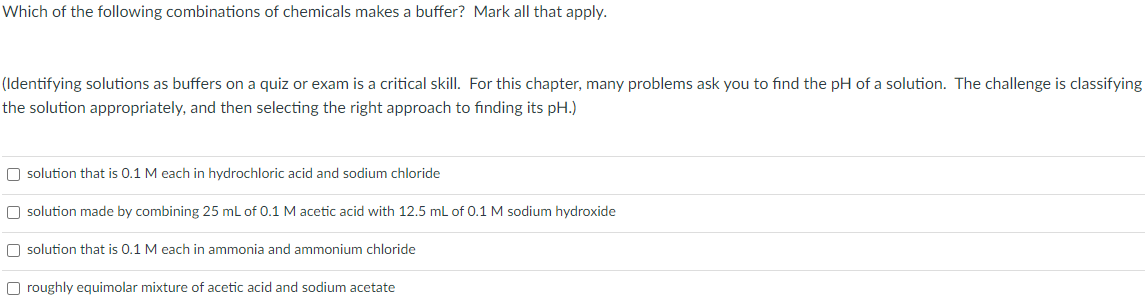
Transcribed Image Text:Which of the following combinations of chemicals makes a buffer? Mark all that apply.
(Identifying solutions as buffers on a quiz or exam is a critical skill. For this chapter, many problems ask you to find the pH of a solution. The challenge is classifying
the solution appropriately, and then selecting the right approach to finding its pH.)
O solution that is 0.1 M each in hydrochloric acid and sodium chloride
O solution made by combining 25 mL of 0.1 M acetic acid with 12.5 mL of 0.1 M sodium hydroxide
O solution that is 0.1 M each in ammonia and ammonium chloride
O roughly equimolar mixture of acetic acid and sodium acetate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning