Which of the following is wrong ? O A) Excitation is defined as the moving of the electrons from lower energy levels to higher levels with certain energy amount O B) Radiation energy is inversely proportional to frequency O C) To move an electron from a 2S orbital to a 35 orbital energy is needed. O D) Electrons in an excited atom emit light at different wavelengths as they move to lower energy levels. O E) If an electron jumps fromn = 3 to n = 2 it gives out as much energy as the difference between them.
Which of the following is wrong ? O A) Excitation is defined as the moving of the electrons from lower energy levels to higher levels with certain energy amount O B) Radiation energy is inversely proportional to frequency O C) To move an electron from a 2S orbital to a 35 orbital energy is needed. O D) Electrons in an excited atom emit light at different wavelengths as they move to lower energy levels. O E) If an electron jumps fromn = 3 to n = 2 it gives out as much energy as the difference between them.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 80QAP: In the photoelectric effect, electrons are ejected from a metal surface when light strikes it. A...
Related questions
Question
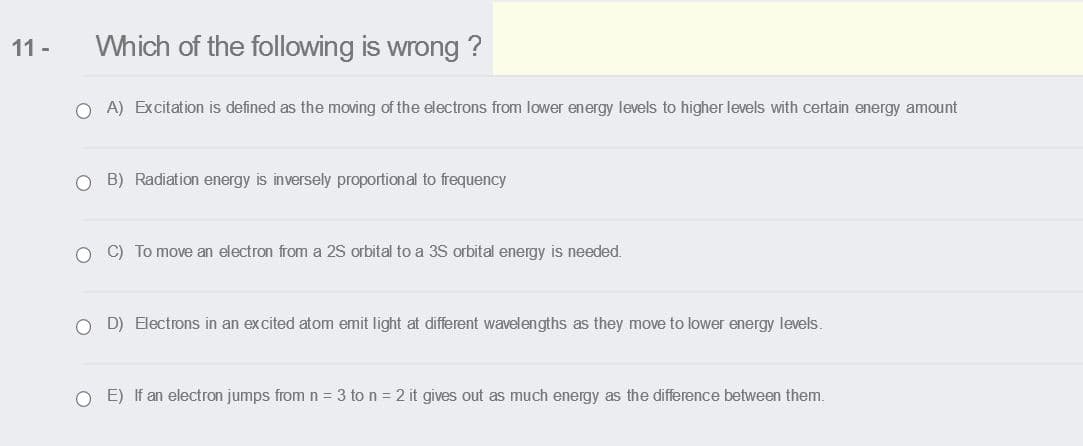
Transcribed Image Text:11 -
Which of the following is wrong ?
O A) Excitation is defined as the moving of the electrons from lower energy levels to higher levels with certain energy amount
O B) Radiation energy is inversely proportional to frequency
O C) To move an electron from a 2S orbital to a 3S orbital energy is needed.
O D) Electrons in an excited atom emit light at different wavelengths as they move to lower energy levels.
O E) If an electron jumps from n = 3 to n = 2 it gives out as much energy as the difference between them.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning