Why are chemical reactions often carried out using solutions? Select all that apply. O The molecules or ions in a solution can freely intermingle. The amount of each reactant can be controlled by adjusting the volume of each solution that is used. O The heterogeneous nature of solutions prevents the reactants from freely mixing. O The solvent must always be water, so it is impossible for a fire to start. O Even very small amounts of reactants can be combined by using dilute solutions. O A color change will always occur when a reaction is conducted in solution.
Why are chemical reactions often carried out using solutions? Select all that apply. O The molecules or ions in a solution can freely intermingle. The amount of each reactant can be controlled by adjusting the volume of each solution that is used. O The heterogeneous nature of solutions prevents the reactants from freely mixing. O The solvent must always be water, so it is impossible for a fire to start. O Even very small amounts of reactants can be combined by using dilute solutions. O A color change will always occur when a reaction is conducted in solution.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 26CR: When a solution is diluted by adding additional solvent, the concentration of solute changes hut the...
Related questions
Question
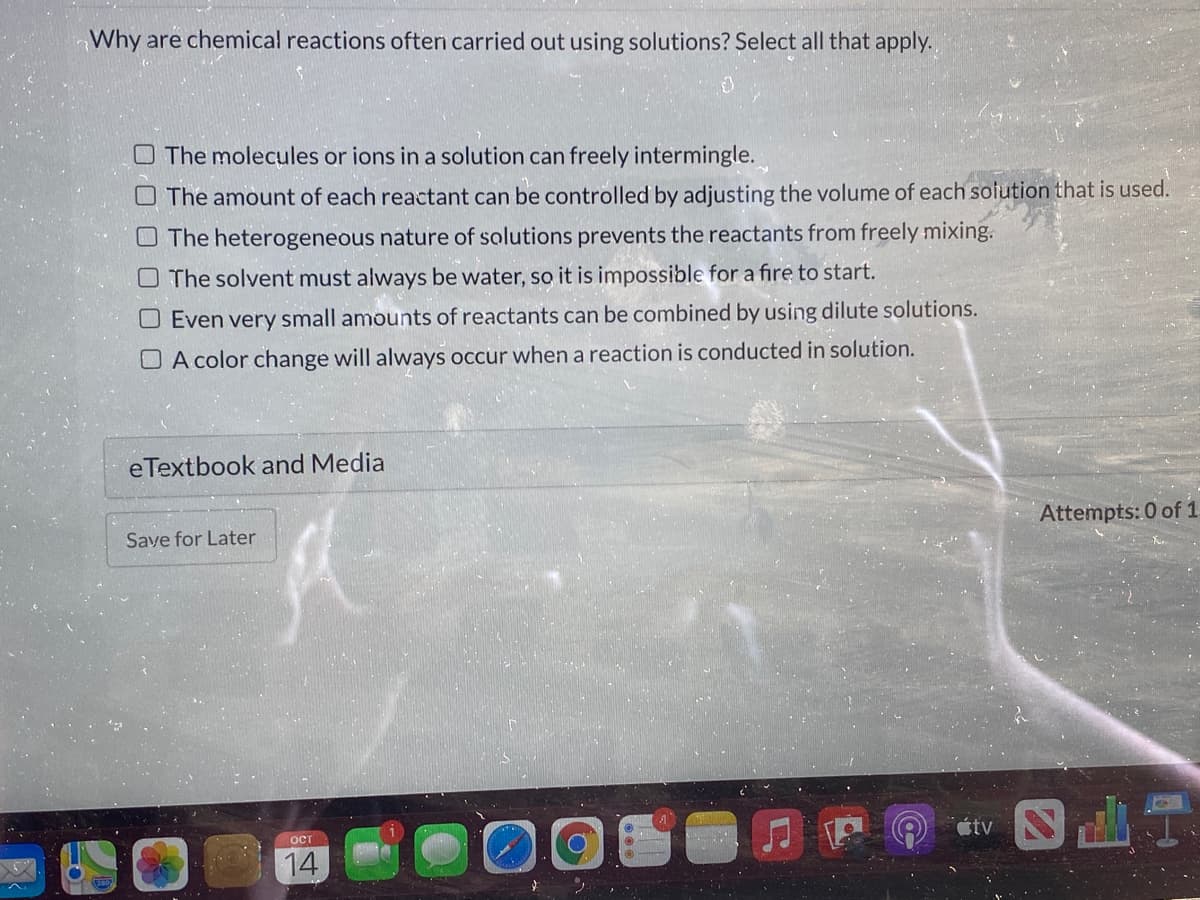
Transcribed Image Text:Why are chemical reactions oftern carried out using solutions? Select all that apply.
O The molecules or ions in a solution can freely intermingle.
O The amount of each reactant can be controlled by adjusting the volume of each solution that is used.
O The heterogeneous nature of solutions prevents the reactants from freely mixing.
O The solvent must always be water, so it is impossible for a fire to start.
O Even very small amounts of reactants can be combined by using dilute solutions.
O A color change will always occur when a reaction is conducted in solution.
eTextbook and Media
Save for Later
Attempts: 0 of 1
O étv
ост
14
4)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning