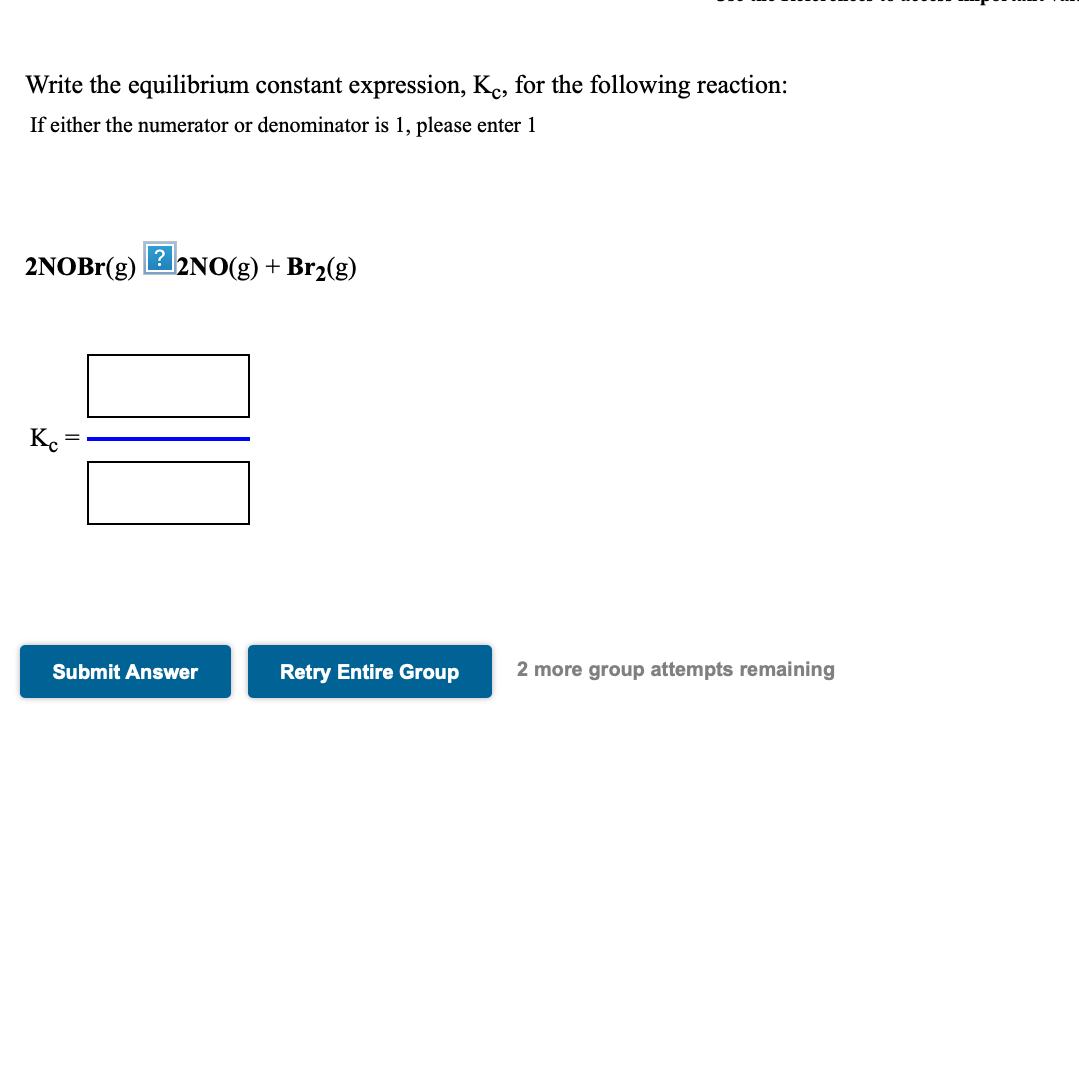
Write the equilibrium constant expression, Kc, for the following reaction: If either the numerator or denominator is 1, please enter 1 2NOBr(g) 2NO(g) + Br2(g)
Write the equilibrium constant expression, Kc, for the following reaction:
| If either the numerator or denominator is 1, please enter 1 |
2NOBr(g) 2NO(g) + Br2(g)
| Kc = | |
Solution
The constant of a chemical change (usually denoted by the image K) provides insight into the connection between the merchandise and reactants once a chemical change reaches equilibrium. for instance, the constant of concentration (denoted by Kc) of a chemical change at equilibrium are often outlined because the quantitative relation of the concentration of merchandise to the concentration of the reactants, every raised to their various ratio coefficients. it's vital to notice that there square measure many differing kinds of equilibrium constants that give relationships between the merchandise and therefore the reactants of equilibrium reactions in terms of various units.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









