Write the letter T or F inside each box after deciding which statement is true or false. Then on the 3rd box, write the letter of your choice based on the given instructions. A if both statements are true. B if the 1st statement is false and the 2nd statement is true. C if the 1st statement is true and
Write the letter T or F inside each box after deciding which statement is true or false. Then on the 3rd box, write the letter of your choice based on the given instructions. A if both statements are true. B if the 1st statement is false and the 2nd statement is true. C if the 1st statement is true and
Chapter5: Chemical Bonding
Section: Chapter Questions
Problem 3SC
Related questions
Question
Write the letter T or F inside each box after deciding which statement is true or false. Then on
the 3rd box, write the letter of your choice based on the given instructions.
A if both statements are true.
B if the 1st statement is false and the 2nd statement is true.
C if the 1st statement is true and the 2nd statement is false.
D if both statements are false.
ANSWER 1-10
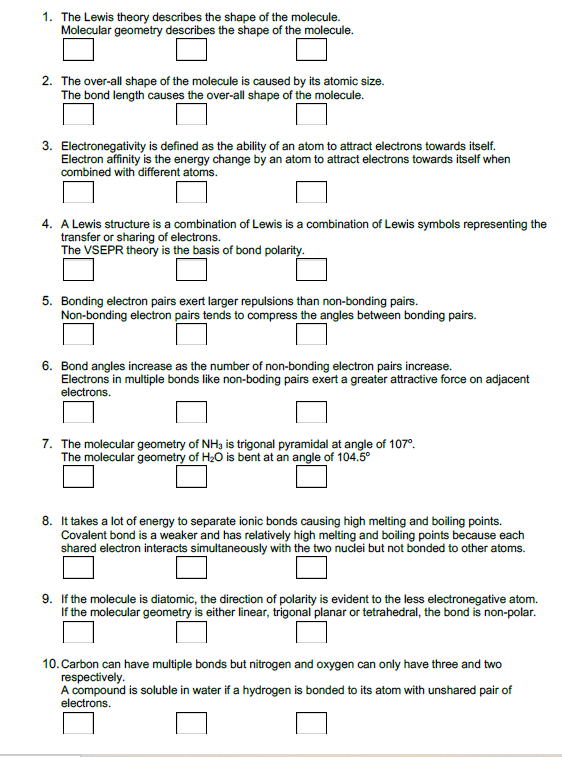
Transcribed Image Text:1. The Lewis theory describes the shape of the molecule.
Molecular geometry describes the shape of the molecule.
2. The over-all shape of the molecule is caused by its atomic size.
The bond length causes the over-all shape of the molecule.
3. Electronegativity is defined as the ability of an atom to attract electrons towards itself.
Electron affinity is the energy change by an atom to attract electrons towards itself when
combined with different atoms.
4. A Lewis structure is a combination of Lewis is a combination of Lewis symbols representing the
transfer or sharing of electrons.
The VSEPR theory is the basis of bond polarity.
5. Bonding electron pairs exert larger repulsions than non-bonding pairs.
Non-bonding electron pairs tends to compress the angles between bonding pairs.
6. Bond angles increase as the number of non-bonding electron pairs increase.
Electrons in multiple bonds like non-boding pairs exert a greater attractive force on adjacent
electrons.
7. The molecular geometry of NH3 is trigonal pyramidal at angle of 107°.
The molecular geometry of H20 is bent at an angle of 104.5°
8. It takes a lot of energy to separate ionic bonds causing high melting and boiling points.
Covalent bond is a weaker and has relatively high melting and boiling points because each
shared electron interacts simultaneously with the two nuclei but not bonded to other atoms.
9. If the molecule is diatomic, the direction of polarity is evident to the less electronegative atom.
molecular geometry is either linear, trigonal planar or tetrahedral, the bond is non-polar.
10. Carbon can have multiple bonds but nitrogen and oxygen can only have three and two
respectively.
A compound is soluble in water if a hydrogen is bonded to its atom with unshared pair of
electrons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning