X bAnswered: Chemistry Question |X (74) How to Write Complete lonic X 101 Chem 101 app.101edu.co Submit Question 37 of 43 Write the balanced COMPLETE ionic equation for the reaction when KOH and Cu(NO3)2 are mixed in aqueous solution. If no reaction occurs, write only NR. 4- |2- |2+ 3+ 4+ + 83 1 2 3 4 5 6 7 0 4 3 17 1 5 (g) (aq) (s) (1) NR Cu Н N K Reset Delete x H20 8:12 PM е OTYPE here to search 11/7/2019 S LO
X bAnswered: Chemistry Question |X (74) How to Write Complete lonic X 101 Chem 101 app.101edu.co Submit Question 37 of 43 Write the balanced COMPLETE ionic equation for the reaction when KOH and Cu(NO3)2 are mixed in aqueous solution. If no reaction occurs, write only NR. 4- |2- |2+ 3+ 4+ + 83 1 2 3 4 5 6 7 0 4 3 17 1 5 (g) (aq) (s) (1) NR Cu Н N K Reset Delete x H20 8:12 PM е OTYPE here to search 11/7/2019 S LO
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 158IP: The vanadium in a sample of ore is converted to VO2+. The VO2+ ion is subsequently titrated with...
Related questions
Question
Transcribed Image Text:X
bAnswered: Chemistry Question |X
(74) How to Write Complete lonic X
101 Chem 101
app.101edu.co
Submit
Question 37 of 43
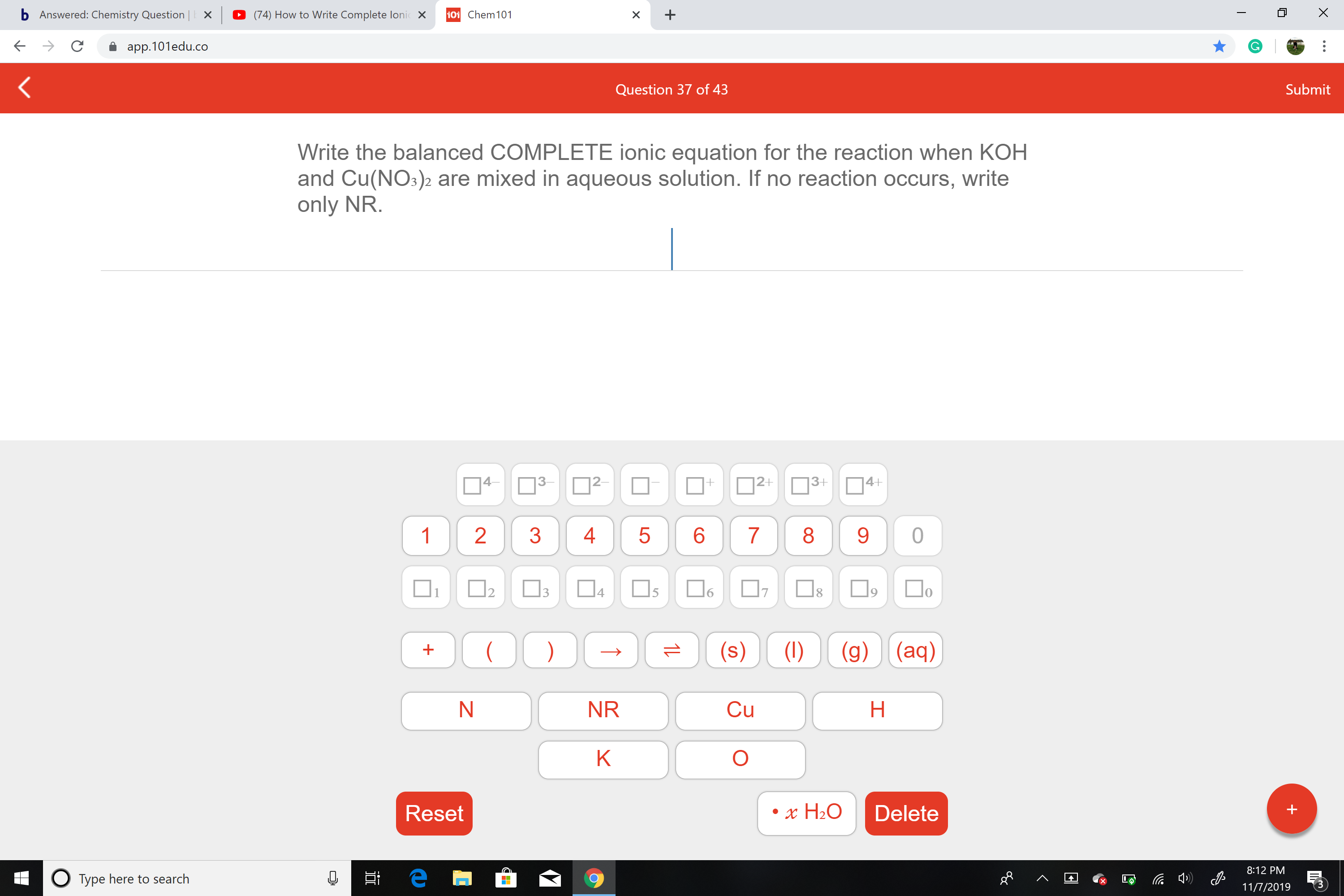
Write the balanced COMPLETE ionic equation for the reaction when KOH
and Cu(NO3)2 are mixed in aqueous solution. If no reaction occurs, write
only NR.
4-
|2-
|2+
3+
4+
+
83
1
2
3
4
5
6
7
0
4
3
17
1
5
(g) (aq)
(s)
(1)
NR
Cu
Н
N
K
Reset
Delete
x H20
8:12 PM
е
OTYPE here to search
11/7/2019
S
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning