x+ Home X CHE101 02: Intro to General Che x X 101 Chem 101 My Questions | bartleby app.101edu.co CUnofficial Transcri... Welcome to the O... myClackamas Login W Logon Oregon Scholarsh... Home - FAFSA on... The National Soc... Apps HW 4.4 Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCI would have to be present to form 17.38 g of MgCl? 7 Mg(OH)2 (s) 2 HCI (aq)2 H2O (I)MgCla (aq) Attempts remaining: 2 7:34 PM Type here to search ENG 11/13/2019
x+ Home X CHE101 02: Intro to General Che x X 101 Chem 101 My Questions | bartleby app.101edu.co CUnofficial Transcri... Welcome to the O... myClackamas Login W Logon Oregon Scholarsh... Home - FAFSA on... The National Soc... Apps HW 4.4 Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCI would have to be present to form 17.38 g of MgCl? 7 Mg(OH)2 (s) 2 HCI (aq)2 H2O (I)MgCla (aq) Attempts remaining: 2 7:34 PM Type here to search ENG 11/13/2019
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.10QAP
Related questions
Question
Transcribed Image Text:x+
Home
X CHE101 02: Intro to General Che x
X
101 Chem 101
My Questions | bartleby
app.101edu.co
CUnofficial Transcri...
Welcome to the O...
myClackamas Login
W Logon
Oregon Scholarsh...
Home - FAFSA on...
The National Soc...
Apps
HW 4.4
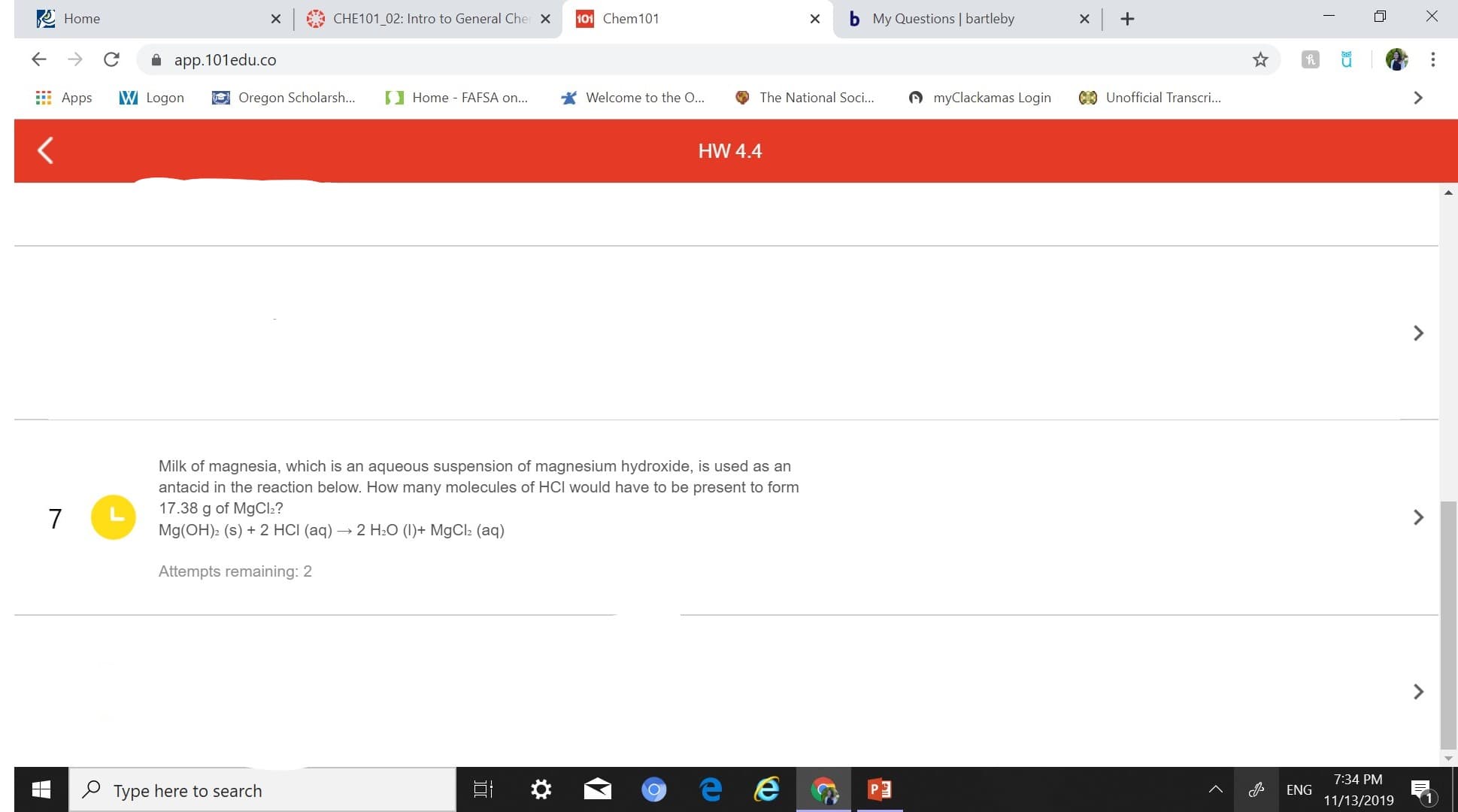
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an
antacid in the reaction below. How many molecules of HCI would have to be present to form
17.38 g of MgCl?
7
Mg(OH)2 (s)
2 HCI (aq)2 H2O (I)MgCla (aq)
Attempts remaining: 2
7:34 PM
Type here to search
ENG
11/13/2019
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
