Rencia- Fall19- PALENCIA Activities and Due Dates> HW 15 Hint Che Resources 985/2200 core: of 22> (aq), which is used frequently in A student needs to prepare 250 mL of a 0.400 M aqueous solution of sucrose, C12H220 biological experiments. 200 mL 250 mL 150 200 100 150 50 250 mL 100 A В Question Source: MRG- General Chemistry | Publishe help terms of use contact us about us privacy policy careers -PalenciaFall9- PALENCIA Activities and Due Dates HW 15 985/2200 nt Score: Che Resources Hint n 12 of 22 How should the correct amount of solute be obtained? Which type of glassware should be used to make this solution (assuming that the accuracy of the concentration Measure out x cm of sucrose with a ruler. is important)? Measure out x mol of solid sucrose on a molemeter. В Measure out xg of solid sucrose on a balance. O A Ос Based on the selected correct unit, what is the value of x? How should the solute and solvent be mixed in the container? Question Source: MRG-General Chemistry | Publis help contact us terms of use privacy policy careers about us 99+
Rencia- Fall19- PALENCIA Activities and Due Dates> HW 15 Hint Che Resources 985/2200 core: of 22> (aq), which is used frequently in A student needs to prepare 250 mL of a 0.400 M aqueous solution of sucrose, C12H220 biological experiments. 200 mL 250 mL 150 200 100 150 50 250 mL 100 A В Question Source: MRG- General Chemistry | Publishe help terms of use contact us about us privacy policy careers -PalenciaFall9- PALENCIA Activities and Due Dates HW 15 985/2200 nt Score: Che Resources Hint n 12 of 22 How should the correct amount of solute be obtained? Which type of glassware should be used to make this solution (assuming that the accuracy of the concentration Measure out x cm of sucrose with a ruler. is important)? Measure out x mol of solid sucrose on a molemeter. В Measure out xg of solid sucrose on a balance. O A Ос Based on the selected correct unit, what is the value of x? How should the solute and solvent be mixed in the container? Question Source: MRG-General Chemistry | Publis help contact us terms of use privacy policy careers about us 99+
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.18QAP
Related questions
Question
Transcribed Image Text:Rencia- Fall19- PALENCIA
Activities and Due Dates> HW 15
Hint
Che
Resources
985/2200
core:
of 22>
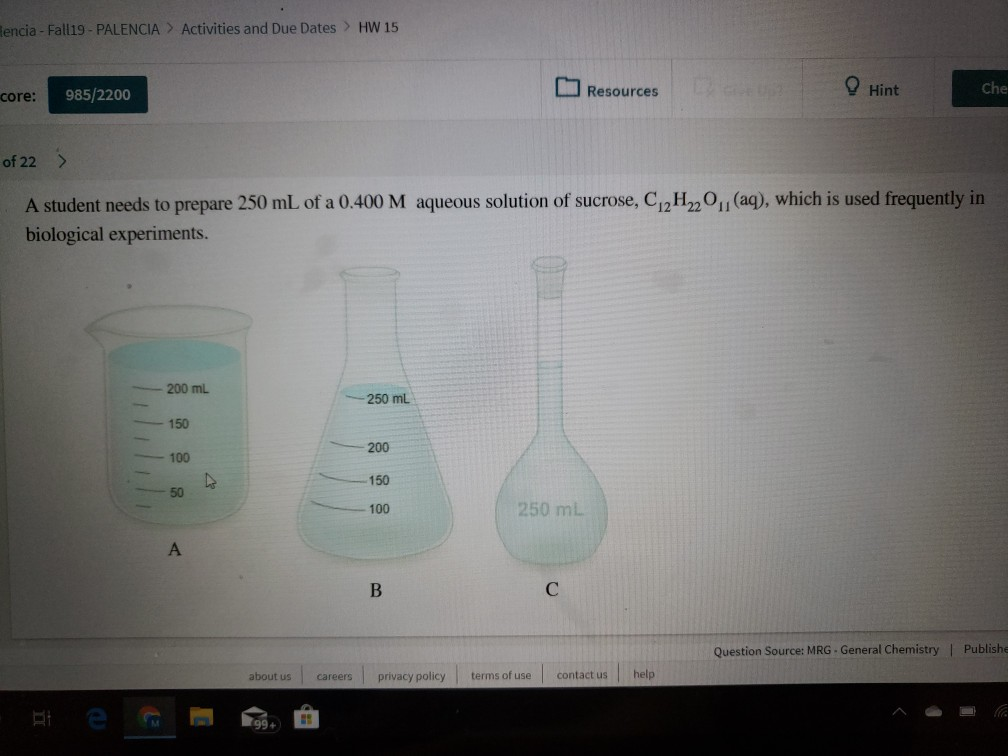
(aq), which is used frequently in
A student needs to prepare 250 mL of a 0.400 M aqueous solution of sucrose, C12H220
biological experiments.
200 mL
250 mL
150
200
100
150
50
250 mL
100
A
В
Question Source: MRG- General Chemistry | Publishe
help
terms of use
contact us
about us
privacy policy
careers
Transcribed Image Text:-PalenciaFall9- PALENCIA
Activities and Due Dates
HW 15
985/2200
nt Score:
Che
Resources
Hint
n 12 of 22
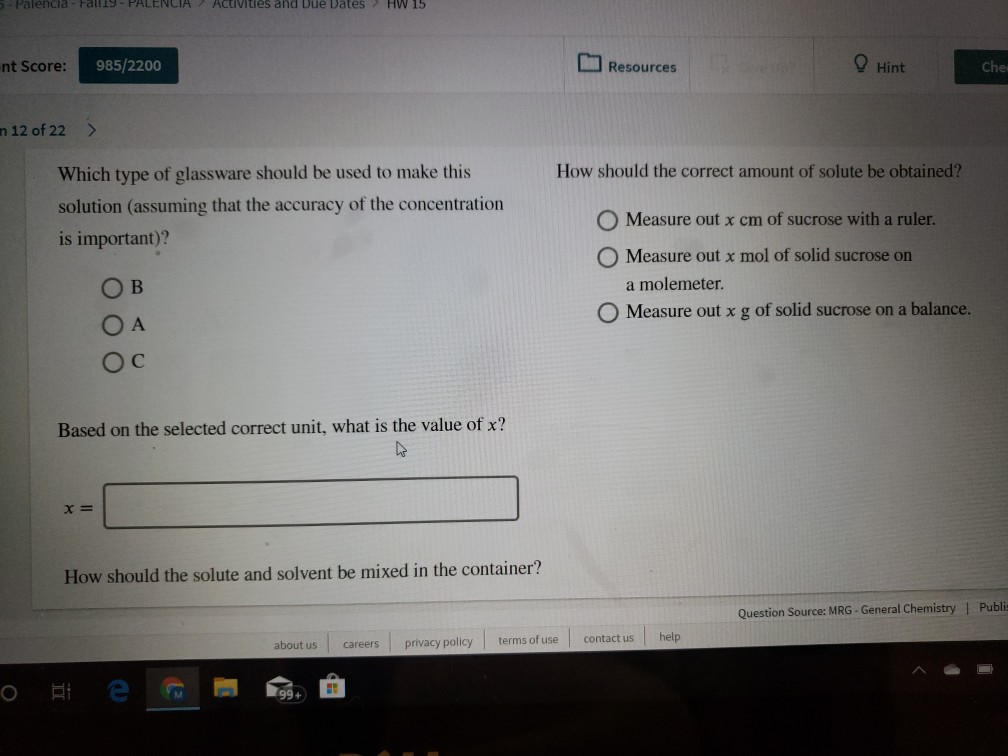
How should the correct amount of solute be obtained?
Which type of glassware should be used to make this
solution (assuming that the accuracy of the concentration
Measure out x cm of sucrose with a ruler.
is important)?
Measure out x mol of solid sucrose on
a molemeter.
В
Measure out xg of solid sucrose on a balance.
O A
Ос
Based on the selected correct unit, what is the value of x?
How should the solute and solvent be mixed in the container?
Question Source: MRG-General Chemistry | Publis
help
contact us
terms of use
privacy policy
careers
about us
99+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

