XeF4 was the first binary compound of a noble gas that was synthesized. It is a square planar molecule and belongs to point group D4h- Considering the sigma bonds of XeF4 as vectors, the following reducible representation was generated. XEF4 Hybridization.pdf L Determine the Hybridization of the sigma bonds of XeF4. Show your solution in the solution sheet and answer the question below.
XeF4 was the first binary compound of a noble gas that was synthesized. It is a square planar molecule and belongs to point group D4h- Considering the sigma bonds of XeF4 as vectors, the following reducible representation was generated. XEF4 Hybridization.pdf L Determine the Hybridization of the sigma bonds of XeF4. Show your solution in the solution sheet and answer the question below.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter13: Introduction To Symmetry In Quantum Mechanics
Section: Chapter Questions
Problem 13.22E: Figure 13.27 shows the structure of the molecule porphine. Figure 13.27 The structure of porphine....
Related questions
Question
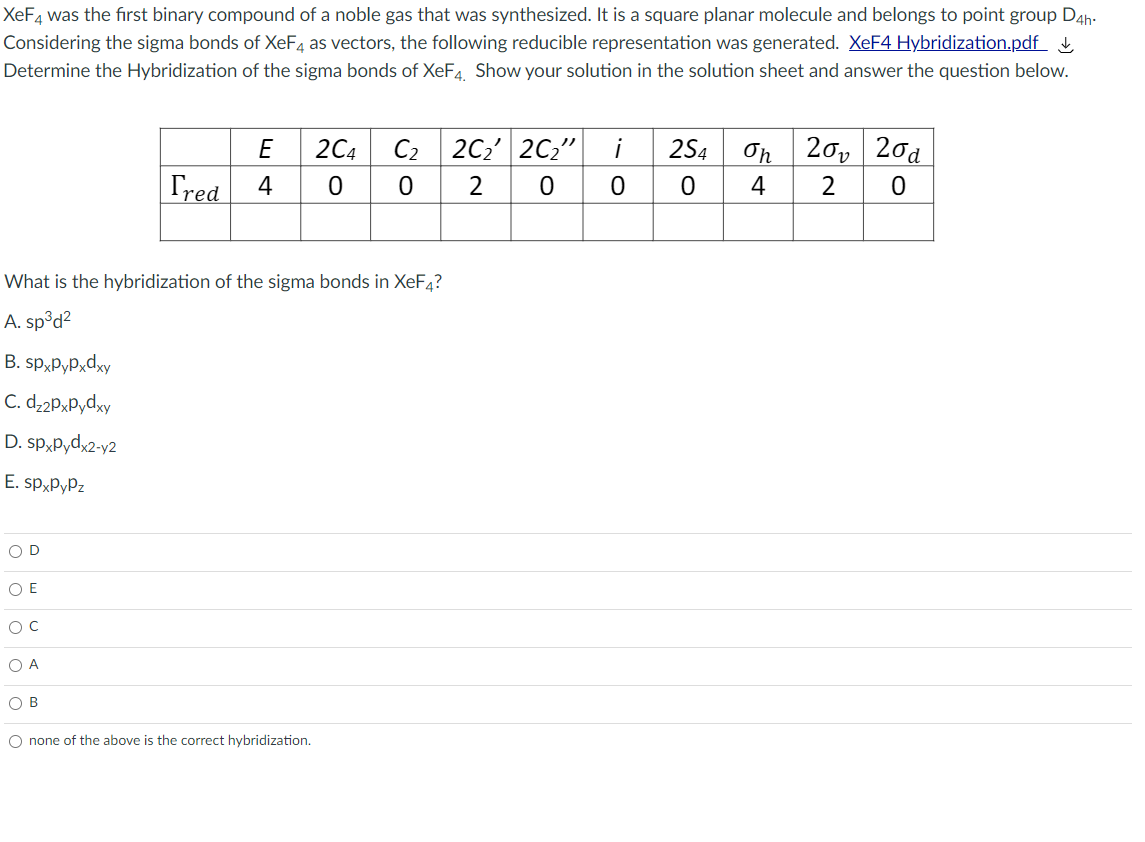
Transcribed Image Text:XeF4 was the first binary compound of a noble gas that was synthesized. It is a square planar molecule and belongs to point group D4h:
Considering the sigma bonds of XeF4 as vectors, the following reducible representation was generated. XEF4 Hybridization.pdf L
Determine the Hybridization of the sigma bonds of XeF4 Show your solution in the solution sheet and answer the question below.
E
2C4
C2 2C2' 2C2" i
2S4
On
20, 20а
Tred
4
2
4
2
What is the hybridization of the sigma bonds in XeF4?
A. sp³d?
Axp*d^d*ds °g
C. d-2PxPydxy
D. sp,Pydx2-y2
E. sp,pyPz
OD
O E
O A
O B
O none of the above is the correct hybridization.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning