y! how to Q What g. y zinc anc y ideal ga y What g Q What g y What g W molarit Experim ourse.html?courseld%3D15828855&HeplD=537061946710f71a192e43597b01c6e0#10301 Lab Lab Part A Why is the KMnO, solution filtered? O KMNO4 doesn't need to be filtered prior to use. O Most commercial samples contain inorganic matter that will react slowly with the KMnO, and cause its decomposition. O KMNO is very stable, but the unstable ions in the commercial packaging materials must be removed prior to use. Most commercial samples of KMnO4 contain some manganese dioxide that must be removed prior to use to avoid further decomposition. Submit Request Answer Part B Why is the KMnO, not stored in a rubber-stoppered bottle? Rubber doesnt make a good seal with the plastic bottles in which permanganate is usually stored. Rubber can oxidize the permanganate solution. P Pearson Copyright 2020 Pearson Education Inc. All righrs reserved. Terms ofUse I Prvacy Policy Permission: 40 DELL
y! how to Q What g. y zinc anc y ideal ga y What g Q What g y What g W molarit Experim ourse.html?courseld%3D15828855&HeplD=537061946710f71a192e43597b01c6e0#10301 Lab Lab Part A Why is the KMnO, solution filtered? O KMNO4 doesn't need to be filtered prior to use. O Most commercial samples contain inorganic matter that will react slowly with the KMnO, and cause its decomposition. O KMNO is very stable, but the unstable ions in the commercial packaging materials must be removed prior to use. Most commercial samples of KMnO4 contain some manganese dioxide that must be removed prior to use to avoid further decomposition. Submit Request Answer Part B Why is the KMnO, not stored in a rubber-stoppered bottle? Rubber doesnt make a good seal with the plastic bottles in which permanganate is usually stored. Rubber can oxidize the permanganate solution. P Pearson Copyright 2020 Pearson Education Inc. All righrs reserved. Terms ofUse I Prvacy Policy Permission: 40 DELL
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.22QAP
Related questions
Question
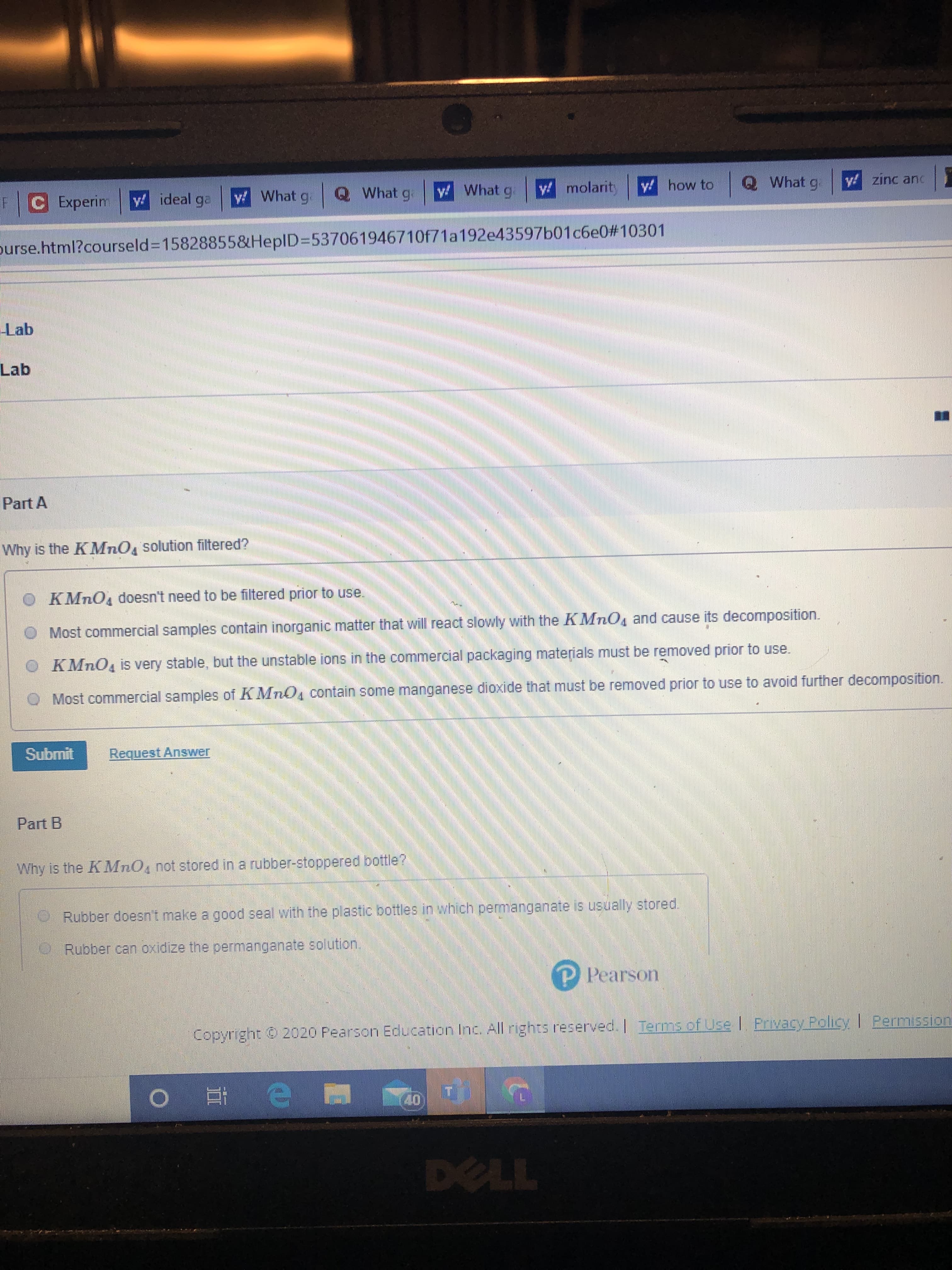
Transcribed Image Text:y! how to
Q What g.
y zinc anc
y ideal ga
y What g
Q What g
y What g
W molarit
Experim
ourse.html?courseld%3D15828855&HeplD=537061946710f71a192e43597b01c6e0#10301
Lab
Lab
Part A
Why is the KMnO, solution filtered?
O KMNO4 doesn't need to be filtered prior to use.
O Most commercial samples contain inorganic matter that will react slowly with the KMnO, and cause its decomposition.
O KMNO is very stable, but the unstable ions in the commercial packaging materials must be removed prior to use.
Most commercial samples of KMnO4 contain some manganese dioxide that must be removed prior to use to avoid further decomposition.
Submit
Request Answer
Part B
Why is the KMnO, not stored in a rubber-stoppered bottle?
Rubber doesnt make a good seal with the plastic bottles in which permanganate is usually stored.
Rubber can oxidize the permanganate solution.
P Pearson
Copyright 2020 Pearson Education Inc. All righrs reserved. Terms ofUse I Prvacy Policy Permission:
40
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning