You have a 25 mL sample of acetylcholine (a neurotransmitter) with an unknown concentration and a pH of 7.93. You incubate this sample with the enzyme acetylcholinesterase to convert all of the acetylcholine to choline and acetic acid. The acetic acid dissociates to yield acetate and hydrogen ions. At the end of the incubation period, you measure the pH again and find that it has decreased to 6.15. Assuming there was no buffer in the assay mixture, determine the number of nanomoles of acetylcholine in the original 25 mL sample. Remember to use significant figures in your answer. CH, CH, сH, —с —о-сн,— сн, — N—сн, H,0 но-сн, —сн,—"N—CH, + сн,—с —о- + н CH, ČH, Acetylcholine Choline Acetate
You have a 25 mL sample of acetylcholine (a neurotransmitter) with an unknown concentration and a pH of 7.93. You incubate this sample with the enzyme acetylcholinesterase to convert all of the acetylcholine to choline and acetic acid. The acetic acid dissociates to yield acetate and hydrogen ions. At the end of the incubation period, you measure the pH again and find that it has decreased to 6.15. Assuming there was no buffer in the assay mixture, determine the number of nanomoles of acetylcholine in the original 25 mL sample. Remember to use significant figures in your answer. CH, CH, сH, —с —о-сн,— сн, — N—сн, H,0 но-сн, —сн,—"N—CH, + сн,—с —о- + н CH, ČH, Acetylcholine Choline Acetate
Biomedical Instrumentation Systems
1st Edition
ISBN:9781133478294
Author:Chatterjee
Publisher:Chatterjee
Chapter6: Biomedical Electrodes, Sensors, And Transducers
Section: Chapter Questions
Problem 3Q
Related questions
Question
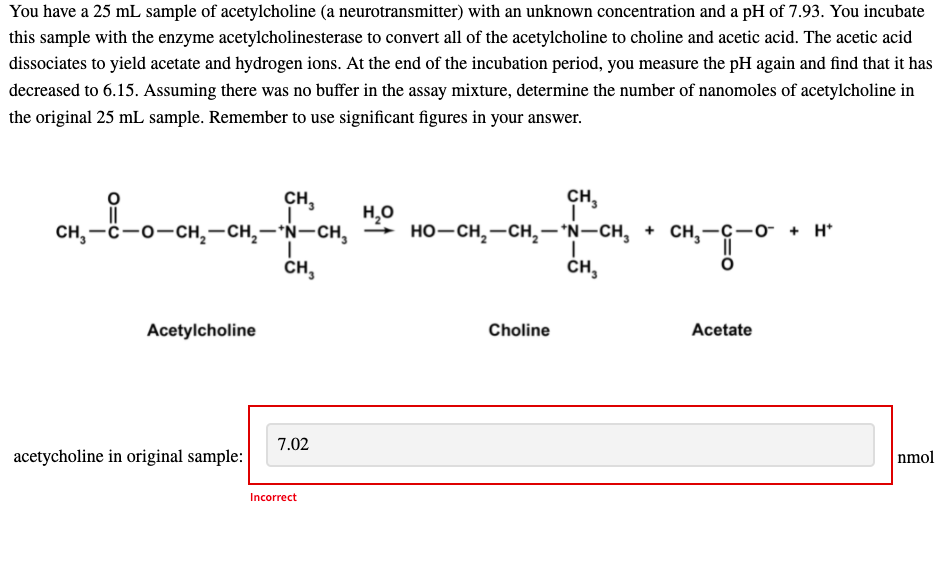
Transcribed Image Text:You have a 25 mL sample of acetylcholine (a neurotransmitter) with an unknown concentration and a pH of 7.93. You incubate
this sample with the enzyme acetylcholinesterase to convert all of the acetylcholine to choline and acetic acid. The acetic acid
dissociates to yield acetate and hydrogen ions. At the end of the incubation period, you measure the pH again and find that it has
decreased to 6.15. Assuming there was no buffer in the assay mixture, determine the number of nanomoles of acetylcholine in
the original 25 mL sample. Remember to use significant figures in your answer.
сн,
CH,
сH, —с —о—сн, — сн, — N—сн,
H,0
но—сн, ——сн,— "N—сн, + сH,—ҫ—о + H
CH,
ČH,
Acetylcholine
Choline
Acetate
7.02
acetycholine in original sample:
nmol
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you



