
Concept explainers
Name each compound and state how many lines are observed in its
a. 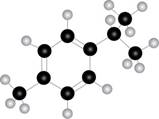 b.
b. 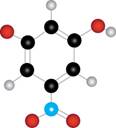
(a)
Interpretation: The name of the given compound is to be determined. The number of lines observed in its
Concept introduction:
Answer to Problem 20P
The IUPAC name of the given compound is
Explanation of Solution
The given molecule is
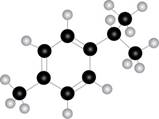
Figure 1
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the compound is,
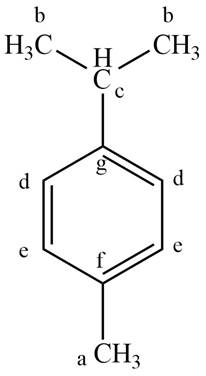
Figure 2
The parent name of the compound is benzene. The numbering will be in alphabetical order. Isopropyl group is present on first carbon atom and methyl group is present on fourth carbon atom. The IUPAC name of the given compound is
Thus, seven lines are present in
The IUPAC name of the given compound is
(b)
Interpretation: The name of the given compound is to be determined. The number of lines observed in its
Concept introduction:
Answer to Problem 20P
The IUPAC name of the given compound is
Explanation of Solution
The given molecule is
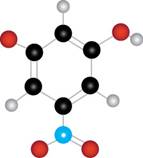
Figure 3
The red coloured balls have two bonds. So, these are the oxygen atoms. The blue coloured ball has three bonds. So, this is the nitrogen atom. The maroon coloured ball has one bond. So, this is the halogen atom. The black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the compound is,
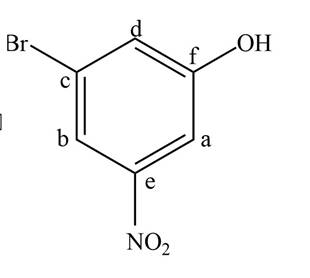
Figure 4
The parent name of the compound is phenol. The numbering will be in alphabetical order. Bromine atom is present on third carbon atom and nitro group is present on fifth carbon atom. The IUPAC name of the given compound is
Thus, six lines are present in
The IUPAC name of the given compound is
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry (6th Edition)
- Explain why the carbonyl carbon of an aldehyde or ketone absorbs farther downeld than the carbonyl carbon of an ester in a 13C NMR spectrum.arrow_forwardHow many peaks would each compound show in their 13C NMR spectrum? a) eucalyptol b) linaloolarrow_forwardName each compound and state how many lines are observed in its 13C NMR spectrum.arrow_forward
- How many 1H NMR signals does each compound show?arrow_forwardExplain why the carbonyl carbon of an aldehyde or ketone absorbs farther downfield than the carbonyl carbon of an ester in a 13C NMR spectrum.arrow_forwardAnswer the following questions about each of the hydroxy ketones: 1-hydroxybutan-2-one (A) and 4-hydroxybutan-2-one (B). a.) How many signals are observed in the 1H NMR spectrum?b.) Give the splitting observed for each type of proton as well as its approximate chemical shift.arrow_forward
- Show its chemical structure through NMR IRarrow_forwardThe 1H NMR chemical shifts of nitromethane, dinitromethane, and trinitromethane are at δ 6.10, δ 4.33, and δ 7.52. Match each chemical shift with the compound. Explain how chemical shift correlates with pKa.arrow_forwardWhich of the diethylbenzene isomers (ortho, meta, or para) correspondsto each set of 13C NMR spectral data ? 13C NMR signals: 16, 29, 128, and 141 ppmarrow_forward
- When heated with chromic acid, compound A forms benzoic acid. Identify compound A from its 1H NMR spectrum.arrow_forwardConsider geraniol, the principal constituent of rose oil.a.How many 1H NMR signals does geraniol exhibit? b.How many 13C NMR signals does geraniol exhibit? c.Into how many peaks will the protons on each C=C be split?arrow_forwardConsider geraniol, the principal constituent of rose oil. a.) How many 1H NMR signals does geraniol exhibit? b.) How many 13C NMR signals does geraniol exhibit?c.) Into how many peaks will the protons on each C=C be split?arrow_forward
