
What would be the effect of carrying out the sodium iodide in acetone reaction with the
Interpretation: The effect of carrying out the sodium iodide in acetone reaction with the alkyl halide using iodide solution half as concentrated is to be interpreted.
Concept introduction: The reaction of alkyl halide with sodium iodide results the formation of alkyl iodide. The reaction occurs in the presence of acetone as the solvent. It is named as Finkelstein reaction and is followed by
Answer to Problem 1Q
In the presence of half concentrated iodide solution, the
Explanation of Solution
The mechanism of the given reaction can be shown as given below:
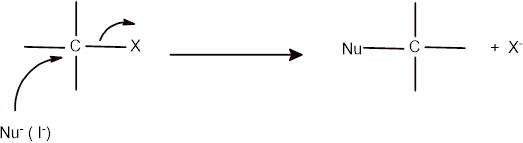
Here the iodide ions act as nucleophile and involve in the rate determining step as reaction follows the
Therefore, with decrease in the concentration of iodide ion, the rate of reaction will also halves that yield only half of the product.
In
Want to see more full solutions like this?
Chapter 17 Solutions
Macroscale and Microscale Organic Experiments
- We used biphenyl instead of benzene for Friedel-Crafts alkylation. What would you predict the major product would be if we had used benzene instead? Why?arrow_forwardIn the Iodination of Acetone, What will happen to the reaction time and reaction rate if the concentration of one of the reactants is doubled while keeping everything the same?arrow_forwardwhat will happen to the reaction of acetone in the sodium nitroprusside Test and in the iodoform test? what will happen if liquid Benzaldehyde is exposed into the air?arrow_forward
- Describe the reason why the pH of the reaction medium affects the rate of coupling of the phenyldiazonium ion with the β-naphthol.arrow_forwardWhich should be more reactive for an SN2 reaction involving sodium iodide in a solution of acetone, 2-chlorobutane or 1-chlorobutane?arrow_forwardWhy is the para substituted derivative formed almost exclusively in the nitration of acetanilide?arrow_forward
- What general conclusion can be made concerning the rate at which the SN2 reaction occurs and the leaving group? 2. Alkyl iodides were not used in this part of the experiment. Why? Explain.arrow_forwardwhy it is important to keep water out of the acid-catalysed esterification of octanoic acid with 1-propanol reaction? What would happen if water got into the reaction mixture?arrow_forwardBased on the theoretical result in the table, what is the order of reactivity of primary, secondary, and tertiary alkyl halides with sodium iodide in acetone?arrow_forward
- Write the mechanism of reaction of benzoic acid with thionyl chloridearrow_forwardWould you expect a change in the frequency for the ester stretch between starting material and product in the nitration of methy benzoate?arrow_forwardWhat is the chemical composition of the nitrating mixture? Why does the incoming nitro group go para and not ortho?arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

