
Concept explainers
Draw the product formed when phenylacetic acid
a.
b.
c.
d.
e.
f.
g.
h.
i.
j.
k.
l.
(a)
Interpretation: The product formed when phenylacetic acid
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
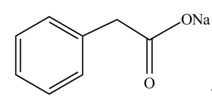
Explanation of Solution
Carboxylic acids react with
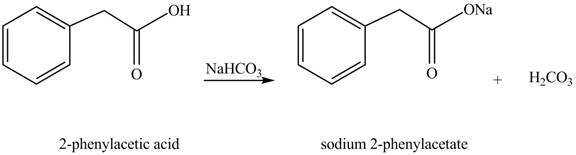
Figure 1
The product formed when phenylacetic acid
(b)
Interpretation: The product formed when phenylacetic acid
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
Explanation of Solution
Carboxylic acids react with
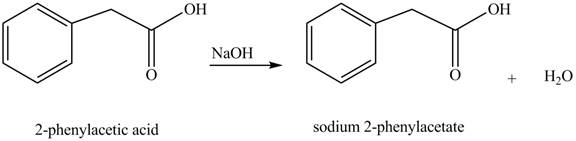
Figure 2
The product formed when phenylacetic acid
(c)
Interpretation: The product formed when phenylacetic acid
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
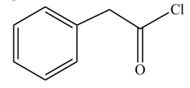
Explanation of Solution
Carboxylic acids react with
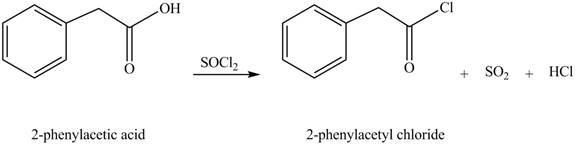
Figure 3
The product formed when phenylacetic acid
(d)
Interpretation: The product formed when phenylacetic acid
Concept introduction: Carboxylic acids does not react with
Answer to Problem 22.45P
No product is formed when phenylacetic acid
Explanation of Solution
Carboxylic acids does not react with
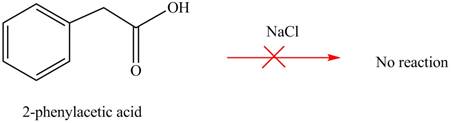
Figure 4
No product is formed when phenylacetic acid
Interpretation: The product formed when phenylacetic acid
(e)
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
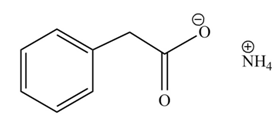
Explanation of Solution
Carboxylic acids react with
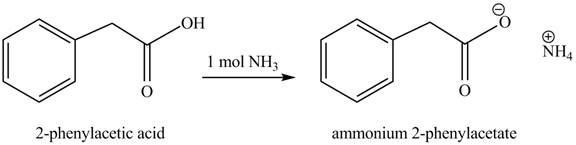
Figure 5
The product formed when phenylacetic acid
Interpretation: The product formed when phenylacetic acid
(f)
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
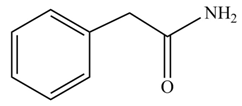
Explanation of Solution
Carboxylic acids react with
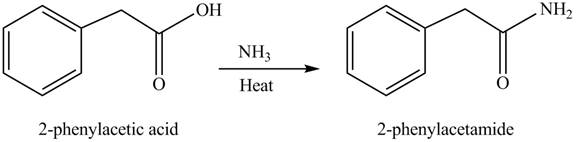
Figure 6
The product formed when phenylacetic acid
Interpretation: The product formed when phenylacetic acid
(g)
Concept introduction: Carboxylic acids react with alcohols in acidic medium to form esters.
Answer to Problem 22.45P
The product formed when phenylacetic acid
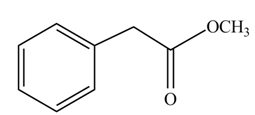
Explanation of Solution
Carboxylic acids react with
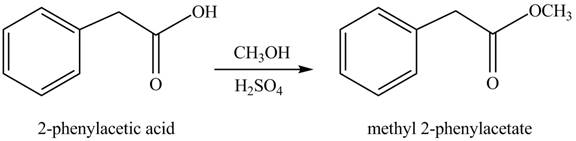
Figure 7
The product formed when phenylacetic acid
Interpretation: The product formed when phenylacetic acid
(h)
Concept introduction: Carboxylic acids react with alcohols in basic medium to form carboxylate ions.
Answer to Problem 22.45P
The product formed when phenylacetic acid
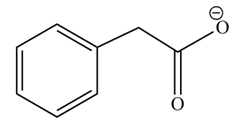
Explanation of Solution
Carboxylic acids react with
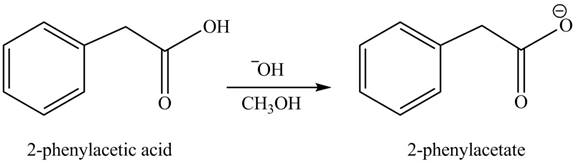
Figure 8
The product formed when phenylacetic acid
Interpretation: The product formed when phenylacetic acid
(i)
Concept introduction: Carboxylic acids react with acid chlorides in presence of strong base to form anhydrides.
Answer to Problem 22.45P
The product formed when phenylacetic acid
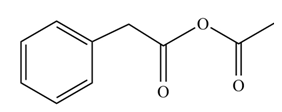
Explanation of Solution
Carboxylic acids react with
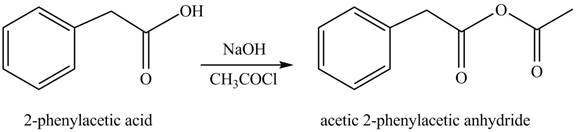
Figure 9
The product formed when phenylacetic acid
Interpretation: The product formed when phenylacetic acid
(j)
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
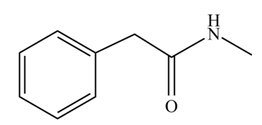
Explanation of Solution
Carboxylic acids react with
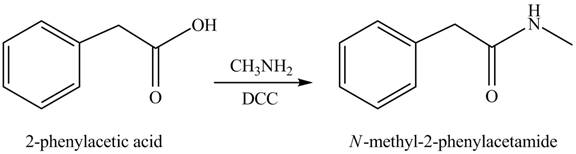
Figure 10
The product formed when phenylacetic acid
Interpretation: The product formed when phenylacetic acid
(k)
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
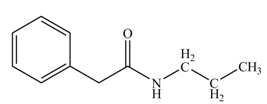
Explanation of Solution
Carboxylic acids react with
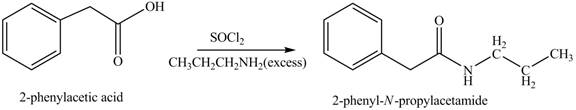
Figure 11
The product formed when phenylacetic acid
(l)
Interpretation: The product formed when phenylacetic acid
Concept introduction: Carboxylic acids react with
Answer to Problem 22.45P
The product formed when phenylacetic acid
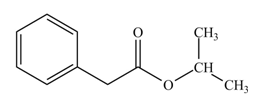
Explanation of Solution
Carboxylic acids react with
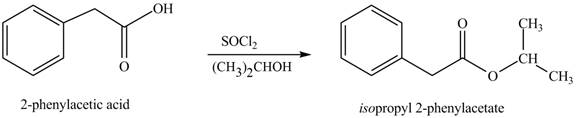
Figure 12
The product formed when phenylacetic acid
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- Thioglycolic acid, HSCH2CO2H, a substance used in depilatory agents (hair removers) has pKa = 3.42. What is the percent dissociation of thioglycolic acid in a buffer solution at pH = 3.0?arrow_forwardan anhydride is reacted with the phenol functional group on salicylic acid in the presence of H2SO4 and heat to produce aspirin, a pain reliever that is not as harsh on the stomach lining as straight salicylic acid. This process is called the a. isomerization of salicylic acid b. dehydration of salicylic acid c. purification of aspirin d. esterification of salicylic acidarrow_forwardDraw the products when phenylacetic acid (C6H5CH2COOH) is treated with each reagent. With some reagents no reaction occurs: A)NaCl B)NH3(1 eqiv) C)1)CH2NH2, 2)CH3COClarrow_forward
- a. Rank the following carboxylic acids from strongest to weakest acid: CH3CH2CH2COOH CH3CH2CHCOOH ClCH2CH2CH2COOH CH3CHCH2COOH b. How does the presence of an electronegative substituent such as Cl affect the acidity of a carboxylic acid? c. How does the location of the substituent affect the acidity of the carboxylic acid?arrow_forwardAmino acids such as glycine are the building blocks of large molecules called proteins that give structure to muscle, tendon, hair, and nails. a. Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion. b. What product is formed when glycine is treated with concentrated HCl? c. What product is formed when glycine is treated with NaOH?arrow_forwardDraw the structure of the predominant form of CF3CH2OH (pK a = 12.4) at pH = 6.arrow_forward
- Draw the product formed when phenylacetic acid (C6H5CH2COOH) is treated with each reagent. With some reagents, no reaction occurs. a. NaHCO3 b. NaOH c. SOCl2 d. NaCl e. NH3(1equiv) f. NH3, ∆ g. CH3OH, H2SO4 h. CH3OH, −OH i. [1] NaOH; [2] CH3COCl j. CH3NH2, DCC k. [1] SOCl2; [2] CH3CH2CH2NH2 (excess) l. [1] SOCl2; [2] (CH3)2; [2] (CHarrow_forwardDraw the products when phenylacetic acid (C6H5CH2COOH) is treated with each reagent. With some reagents no reaction occurs: a)NH3,∆ B)1) SOCl, 2) CH3CH2CH2NH2 (excess) C) 1)SOCl2, 2) (CH3)2CHOHarrow_forwardDraw the products formed from the acid–base reaction of H2SO4 with each compound.arrow_forward
- Amino acids such as glycine are the building blocks of large molecule.called proteins that give structure to muscle, tendon, hair, and nails.a.Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion. b.What product is formed when glycine is treated with concentrated HCl? c. What product is formed when glycine is treated with NaOH?arrow_forward(C₈H₁₀) according to the following reaction: C₈H₁₀(l) + O₂(g) + NH₃(g) → C₈H₄N₂(s) + H₂O(l) How many grams of water would be produced by the complete ammoxidation of 16.50 moles of o-xylene?arrow_forwardplease find Ka values of Succinic Acid (C4H6O4)arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


