
Concept explainers
(a)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between
(a)
Answer to Problem 6.136EP
Nitrogen-containing compound that is required was,
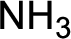
Explanation of Solution
Given structure of compound is,
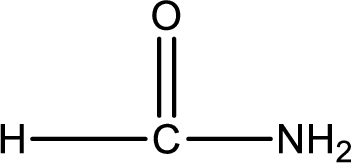
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
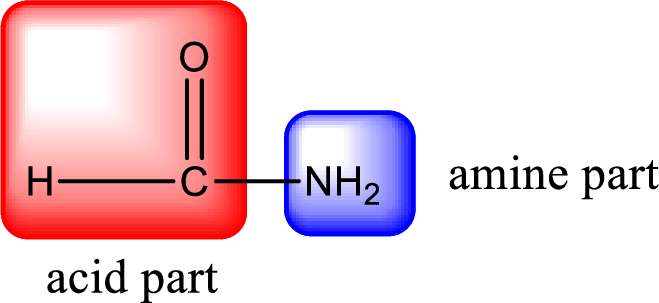
Hydrogen atom has to be added to the amine part and
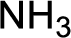
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(b)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(b)
Answer to Problem 6.136EP
Nitrogen-containing compound that is required was,
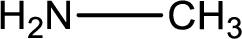
Explanation of Solution
Given structure of compound is,
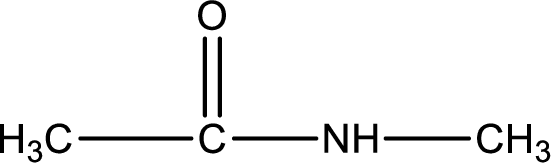
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
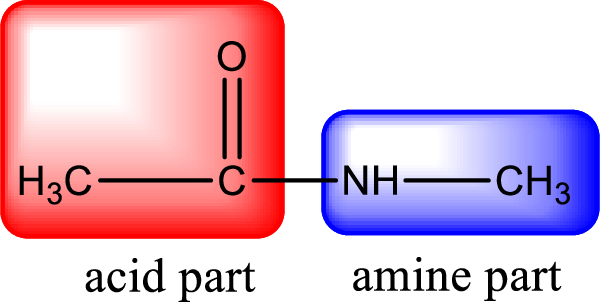
Hydrogen atom has to be added to the amine part and
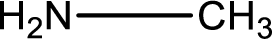
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(c)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(c)
Answer to Problem 6.136EP
Nitrogen-containing compound that is required was,
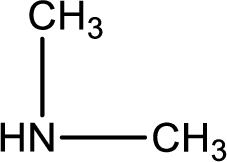
Explanation of Solution
Given structure of compound is,
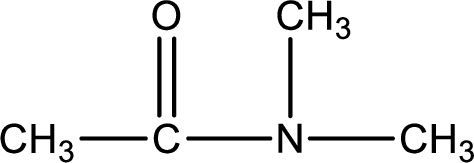
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
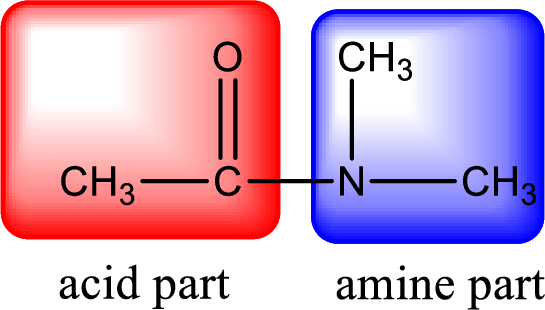
Hydrogen atom has to be added to the amine part and
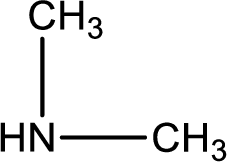
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
(d)
Interpretation:
Structure of nitrogen-containing compound that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the
(d)
Answer to Problem 6.136EP
Nitrogen-containing compound that is required was,
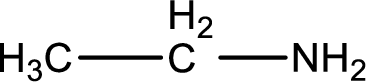
Explanation of Solution
Given structure of compound is,
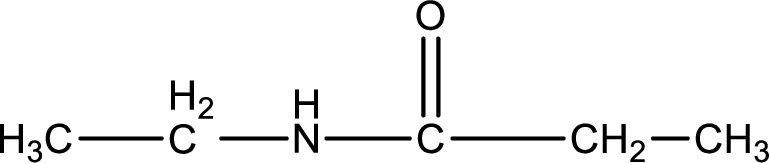
As the nitrogen atom present in the above amide has two hydrogen atoms bonded to it, the amide is a primary amide. Primary amide is produced by the reaction of ammonia with carboxylic acid. The nitrogen-containing compound can be identified as shown below,
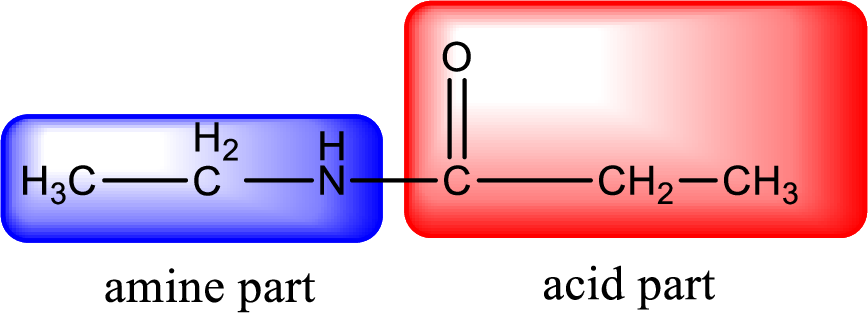
Hydrogen atom has to be added to the amine part and
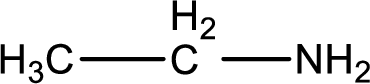
Structure of nitrogen-containing compound that is required to produce the given compound is drawn.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic And Biological Chemistry
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

